Streptolysin O Induces the Ubiquitination and Degradation of Pro-IL-1β
- PMID: 30889575
- PMCID: PMC6758947
- DOI: 10.1159/000496403
Streptolysin O Induces the Ubiquitination and Degradation of Pro-IL-1β
Abstract
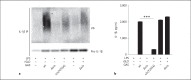
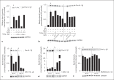
Group A Streptococcus (GAS) is a common and versatile human pathogen causing a variety of diseases. One of the many virulence factors of GAS is the secreted pore-forming cytotoxin streptolysin O (SLO), which has been ascribed multiple properties, including inflammasome activation leading to release of the potent inflammatory cytokine IL-1β from infected macrophages. IL-1β is synthesized as an inactive pro-form, which is activated intracellularly through proteolytic cleavage. Here, we use a macrophage infection model to show that SLO specifically induces ubiquitination and degradation of pro-IL-1β. Ubiquitination was dependent on SLO being released from the infecting bacterium, and pore formation by SLO was required but not sufficient for the induction of ubiquitination. Our data provide evidence for a novel SLO-mediated mechanism of immune regulation, emphasizing the importance of this pore-forming toxin in bacterial virulence and pathogenesis.
Keywords: Group A Streptococcus; Streptolysin O; Ubiquitin, IL-1β.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures



References
-
- Hafner-Bratkovič I, Pelegrín P. Ion homeostasis and ion channels in NLRP3 inflammasome activation and regulation. Curr Opin Immunol. 2018 Jun;52:8–17. - PubMed
-
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015 Oct;526((7575)):660–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

