Synthesis of multi ring-fused imidazo [1,2- a]isoquinoline-based fluorescent scaffold as anti-Herpetic agent
- PMID: 30889631
- PMCID: PMC5890510
- DOI: 10.1177/2040206616680968
Synthesis of multi ring-fused imidazo [1,2- a]isoquinoline-based fluorescent scaffold as anti-Herpetic agent
Abstract
Background: Natural product-inspired synthesis is a key incorporation in modern diversity-oriented synthesis to yield biologically novel scaffold. Inspired by β-carboline fused system, we have designed molecules with multi ring fused scaffold by modifying the tricyclic pyrido[3,4- b]indole ring with imidazo[1,2- a]isoquinoline.
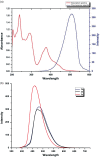
Methods: A highly convergent approach with new C-N and C-C bond formation to synthesize multiring fused complex scaffold imidazo[1,2- a]isoquinolinies as fluorophores. N-nucleophile-induced ring transformation of 2 H-pyran-2-one followed by in situ cis-stilbene-type oxidative photocyclization yielded new C-C bond formation without additional oxidant. The cytotoxicity, effective concentrations, and the mode of action of the synthesized analogs were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),, plaque reduction, time of addition, and reverse transcriptase Polymerase Chain Reaction (PCR).
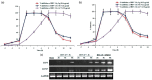
Results: Novel imidazo[1,2- a]isoquinoline analogs were prepared, and the results revealed that trans isomer of cyclopropyl analog (EC50 35 and 37.5 µg/ml) and trans isomer of citric acid salt of phenyl analog (EC50 38.2 and 39.8 µg/ml) possess significant anti-Herpes Simplex Virus (HSV) activity with selectivity index of >10. The kinetic study demonstrated that both the analogs inhibited HSV-1F and HSV-2G at 2-4 h postinfection. Finally, western blot and reverse transcriptase PCR assays revealed that both the analogs suppressed viral immediate early transcription.
Conclusion: Novel imidazo[1,2- a]isoquinoline analogs were synthesized from pyranone with appropriate amines. Two compounds showed better antiviral profile on HSV-infected Vero cells, compared to the standard drug acyclovir (ACV). Overall, we discovered a promising scaffold to develop a nonnucleoside lead targeting the viral immediate early transcription for the management of HSV infections.
Keywords: 2-]isoquinoline; Imidazo[1; herpes simplex virus; immediate early transcription.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Synthesis and antiviral activity of new phenylimidazopyridines and N-benzylidenequinolinamines derived by molecular simplification of phenylimidazo[4,5-g]quinolines.Eur J Med Chem. 2014 Sep 12;84:8-16. doi: 10.1016/j.ejmech.2014.07.011. Epub 2014 Jul 4. Eur J Med Chem. 2014. PMID: 25014745
-
Synthesis, structure-activity relationship and in vitro biological evaluation of N-arylethyl isoquinoline derivatives as Coxsackievirus B3 inhibitors.Bioorg Med Chem Lett. 2011 Oct 1;21(19):5787-90. doi: 10.1016/j.bmcl.2011.08.002. Epub 2011 Aug 8. Bioorg Med Chem Lett. 2011. PMID: 21880491
-
Synthesis, antiproliferative and antiviral activity of imidazo[4,5-d]isothiazole nucleosides as 5:5 fused analogs of nebularine and 6-methylpurine ribonucleoside.J Med Chem. 1997 Feb 28;40(5):771-84. doi: 10.1021/jm960605z. J Med Chem. 1997. PMID: 9057864
-
[Herpes simplex virus latency, reactivation, and a new antiviral therapy for herpetic keratitis].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):247-64; discussion 265. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411713 Review. Japanese.
-
New strategies against drug resistance to herpes simplex virus.Int J Oral Sci. 2016 Mar 30;8(1):1-6. doi: 10.1038/ijos.2016.3. Int J Oral Sci. 2016. PMID: 27025259 Free PMC article. Review.
Cited by
-
Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications.Molecules. 2024 Jun 5;29(11):2668. doi: 10.3390/molecules29112668. Molecules. 2024. PMID: 38893542 Free PMC article. Review.
References
-
- Chen J, Li W, Yao H, et al. Insights into drug discovery from natural products through structural modification. Fitoterapia 2015; 103: 231–241. - PubMed
-
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the Last 25 years. J Nat Prod 2007; 70: 461–467. - PubMed
-
- Paterson I, Anderson EA. The renaissance of natural products as drug candidates. Science 2005; 310: 451. - PubMed
-
- Cao R, Peng W, Wang Z, et al. β-Carboline alkaloids: biochemical and pharmacological functions. Curr Med Chem 2007; 14: 479–500. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

