Choline Supplementation in Cystic Fibrosis-The Metabolic and Clinical Impact
- PMID: 30889905
- PMCID: PMC6471815
- DOI: 10.3390/nu11030656
Choline Supplementation in Cystic Fibrosis-The Metabolic and Clinical Impact
Abstract
Background: Choline is essential for the synthesis of liver phosphatidylcholine (PC), parenchymal maintenance, bile formation, and lipoprotein assembly to secrete triglycerides. In choline deficiency, the liver accretes choline/PC at the expense of lung tissue, thereby impairing pulmonary PC homoeostasis. In cystic fibrosis (CF), exocrine pancreas insufficiency results in impaired cleavage of bile PC and subsequent fecal choline loss. In these patients, the plasma choline concentration is low and correlates with lung function. We therefore investigated the effect of choline supplementation on plasma choline/PC concentration and metabolism, lung function, and liver fat.
Methods: 10 adult male CF patients were recruited (11/2014⁻1/2016), and orally supplemented with 3 × 1 g choline chloride for 84 (84⁻91) days. Pre-/post-supplementation, patients were spiked with 3.6 mg/kg [methyl-D₉]choline chloride to assess choline/PC metabolism. Mass spectrometry, spirometry, and hepatic nuclear resonance spectrometry served for analysis.
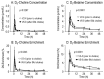
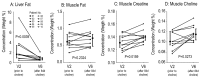
Results: Supplementation increased plasma choline from 4.8 (4.1⁻6.2) µmol/L to 10.5 (8.5⁻15.5) µmol/L at d84 (p < 0.01). Whereas plasma PC concentration remained unchanged, D₉-labeled PC was decreased (12.2 [10.5⁻18.3] µmol/L vs. 17.7 [15.5⁻22.4] µmol/L, p < 0.01), indicating D₉-tracer dilution due to higher choline pools. Supplementation increased Forced Expiratory Volume in 1 second percent of predicted (ppFEV1) from 70.0 (50.9⁻74.8)% to 78.3 (60.1⁻83.9)% (p < 0.05), and decreased liver fat from 1.58 (0.37⁻8.82)% to 0.84 (0.56⁻1.17)% (p < 0.01). Plasma choline returned to baseline concentration within 60 h.
Conclusions: Choline supplementation normalized plasma choline concentration and increased choline-containing PC precursor pools in adult CF patients. Improved lung function and decreased liver fat suggest that in CF correcting choline deficiency is clinically important. Choline supplementation of CF patients should be further investigated in randomized, placebo-controlled trials.
Keywords: choline deficiency; choline supplementation; cystic fibrosis; liver; lung function; magnetic resonance spectroscopy; stable isotope labeling; steatosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Low Plasma Choline, High Trimethylamine Oxide, and Altered Phosphatidylcholine Subspecies Are Prevalent in Cystic Fibrosis Patients with Pancreatic Insufficiency.Nutrients. 2025 Feb 28;17(5):868. doi: 10.3390/nu17050868. Nutrients. 2025. PMID: 40077735 Free PMC article.
-
Plasma phosphatidylcholine alterations in cystic fibrosis patients: impaired metabolism and correlation with lung function and inflammation.Cell Physiol Biochem. 2015;35(4):1437-53. doi: 10.1159/000373964. Epub 2015 Mar 12. Cell Physiol Biochem. 2015. PMID: 25791258
-
Choline Supplementation With a Structured Lipid in Children With Cystic Fibrosis: A Randomized Placebo-Controlled Trial.J Pediatr Gastroenterol Nutr. 2016 Apr;62(4):618-26. doi: 10.1097/MPG.0000000000001004. J Pediatr Gastroenterol Nutr. 2016. PMID: 26465792 Free PMC article. Clinical Trial.
-
Choline in cystic fibrosis: relations to pancreas insufficiency, enterohepatic cycle, PEMT and intestinal microbiota.Eur J Nutr. 2021 Jun;60(4):1737-1759. doi: 10.1007/s00394-020-02358-2. Epub 2020 Aug 14. Eur J Nutr. 2021. PMID: 32797252 Review.
-
Choline and choline-related nutrients in regular and preterm infant growth.Eur J Nutr. 2019 Apr;58(3):931-945. doi: 10.1007/s00394-018-1834-7. Epub 2018 Oct 8. Eur J Nutr. 2019. PMID: 30298207 Review.
Cited by
-
The Effect of Yucca schidigera Extract on Serum Metabolites of Angus Crossbreed Steers with Metabolomics.Metabolites. 2024 Jan 15;14(1):58. doi: 10.3390/metabo14010058. Metabolites. 2024. PMID: 38248861 Free PMC article.
-
Choline supplements: An update.Front Endocrinol (Lausanne). 2023 Mar 7;14:1148166. doi: 10.3389/fendo.2023.1148166. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36950691 Free PMC article. Review.
-
A blend of medium-chain fatty acids, butyrate, organic acids, and a phenolic compound accelerates microbial maturation in newly weaned piglets.PLoS One. 2023 Jul 28;18(7):e0289214. doi: 10.1371/journal.pone.0289214. eCollection 2023. PLoS One. 2023. PMID: 37506070 Free PMC article.
-
Low Plasma Choline, High Trimethylamine Oxide, and Altered Phosphatidylcholine Subspecies Are Prevalent in Cystic Fibrosis Patients with Pancreatic Insufficiency.Nutrients. 2025 Feb 28;17(5):868. doi: 10.3390/nu17050868. Nutrients. 2025. PMID: 40077735 Free PMC article.
-
Choline Kinetics in Neonatal Liver, Brain and Lung-Lessons from a Rodent Model for Neonatal Care.Nutrients. 2022 Feb 8;14(3):720. doi: 10.3390/nu14030720. Nutrients. 2022. PMID: 35277079 Free PMC article.
References
-
- Chen A.H., Innis S.M., Davidson A.G., James S.J. Phosphatidylcholine and lysophosphatidylcholine excretion is increased in children with cystic fibrosis and is associated with plasma homocysteine, S-adenosylhomocysteine, and S-adenosylmethionine. Am. J. Clin. Nutr. 2005;81:686–691. doi: 10.1093/ajcn/81.3.686. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

