Interleukin-1 alpha increases anti-tumor efficacy of cetuximab in head and neck squamous cell carcinoma
- PMID: 30890189
- PMCID: PMC6425573
- DOI: 10.1186/s40425-019-0550-z
Interleukin-1 alpha increases anti-tumor efficacy of cetuximab in head and neck squamous cell carcinoma
Abstract
Background: Despite the high prevalence of epidermal growth factor receptor (EGFR) overexpression in head and neck squamous cell carcinomas (HNSCCs), incorporation of the EGFR inhibitor cetuximab into the clinical management of HNSCC has not led to significant changes in long-term survival outcomes. Therefore, the identification of novel therapeutic approaches to enhance the clinical efficacy of cetuximab could lead to improved long-term survival for HNSCC patients. Our previous work suggests that EGFR inhibition activates the interleukin-1 (IL-1) pathway via tumor release of IL-1 alpha (IL-1α), although the clinical implications of activating this pathway are unclear in the context of cetuximab therapy. Given the role of IL-1 signaling in anti-tumor immune response, we hypothesized that increases in IL-1α levels would enhance tumor response to cetuximab.
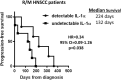
Methods: Parental and stable myeloid differentiation primary response gene 88 (MyD88) and IL-1 receptor 1 (IL-1R1) knockdown HNSCC cell lines, an IL-1R antagonist (IL-1RA), neutralizing antibodies to IL-1α and IL-1β, and recombinant IL-1α and IL-1β were used to determine cytokine production (using ELISA) in response to cetuximab in vitro. IL-1 pathway modulation in mouse models was accomplished by administration of IL-1RA, stable overexpression of IL-1α in SQ20B cells, administration of rIL-1α, and administration of a polyanhydride nanoparticle formulation of IL-1α. CD4+ and CD8+ T cell-depleting antibodies were used to understand the contribution of T cell-dependent anti-tumor immune responses. Baseline serum levels of IL-1α were measured using ELISA from HNSCC patients treated with cetuximab-based therapy and analyzed for association with progression free survival (PFS).
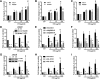
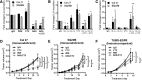
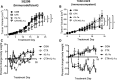
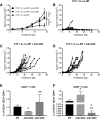
Results: Cetuximab induced pro-inflammatory cytokine secretion from HNSCC cells in vitro which was mediated by an IL-1α/IL-1R1/MyD88-dependent signaling pathway. IL-1 signaling blockade did not affect the anti-tumor efficacy of cetuximab, while increased IL-1α expression using polyanhydride nanoparticles in combination with cetuximab safely and effectively induced a T cell-dependent anti-tumor immune response. Detectable baseline serum levels of IL-1α were associated with a favorable PFS in cetuximab-based therapy-treated HNSCC patients compared to HNSCC patients with undetectable levels.
Conclusions: Altogether, these results suggest that IL-1α in combination with cetuximab can induce a T cell-dependent anti-tumor immune response and may represent a novel immunotherapeutic strategy for EGFR-positive HNSCCs.
Keywords: Anakinra; Biomarker; Cetuximab; Cytokines; EGFR; HNSCC; Interleukin-1; Nanoparticle.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
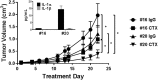

References
-
- Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
