Ablation of elongation factor 2 kinase enhances heat-shock protein 90 chaperone expression and protects cells under proteotoxic stress
- PMID: 30890561
- PMCID: PMC6509509
- DOI: 10.1074/jbc.AC119.008036
Ablation of elongation factor 2 kinase enhances heat-shock protein 90 chaperone expression and protects cells under proteotoxic stress
Abstract
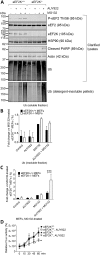
Eukaryotic elongation factor 2 kinase (eEF2K) negatively regulates the elongation stage of mRNA translation and is activated under different stress conditions to slow down protein synthesis. One effect of eEF2K is to alter the repertoire of expressed proteins, perhaps to aid survival of stressed cells. Here, we applied pulsed stable isotope labeling with amino acids in cell culture (SILAC) to study changes in the synthesis of specific proteins in human lung adenocarcinoma (A549) cells in which eEF2K had been depleted by an inducible shRNA. We discovered that levels of heat-shock protein 90 (HSP90) are increased in eEF2K-depleted human cells as well as in eEF2K-knockout (eEF2K-/-) mouse embryonic fibroblasts (MEFs). This rise in HSP90 coincided with an increase in the fraction of HSP90 mRNAs associated with translationally active polysomes, irrespective of unchanged total HSP90 levels. These results indicate that blocking eEF2K function can enhance expression of HSP90 chaperones. In eEF2K-/- mouse embryonic fibroblasts (MEFs), inhibition of HSP90 by its specific inhibitor AUY922 promoted the accumulation of ubiquitinated proteins. Notably, HSP90 inhibition promoted apoptosis of eEF2K-/- MEFs under proteostatic stress induced by the proteasome inhibitor MG132. Up-regulation of HSP90 likely protects cells from protein folding stress, arising, for example, from faster rates of polypeptide synthesis due to the lack of eEF2K. Our findings indicate that eEF2K and HSPs closely cooperate to maintain proper proteostasis and suggest that concomitant inhibition of HSP90 and eEF2K could be a strategy to decrease cancer cell survival.
Keywords: apoptosis; cancer; elongation; eukaryotic elongation factor 2 kinase (eEF2K); heat shock protein (HSP); mRNA translation; molecular chaperone; proteasome; protein synthesis; translation elongation factor.
© 2019 Xie et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Eukaryotic elongation factor 2 kinase upregulates the expression of proteins implicated in cell migration and cancer cell metastasis.Int J Cancer. 2018 May 1;142(9):1865-1877. doi: 10.1002/ijc.31210. Epub 2017 Dec 27. Int J Cancer. 2018. PMID: 29235102
-
eEF2K enhances expression of PD-L1 by promoting the translation of its mRNA.Biochem J. 2020 Nov 27;477(22):4367-4381. doi: 10.1042/BCJ20200697. Biochem J. 2020. PMID: 33094805
-
Direct and indirect activation of eukaryotic elongation factor 2 kinase by AMP-activated protein kinase.Cell Signal. 2017 Aug;36:212-221. doi: 10.1016/j.cellsig.2017.05.010. Epub 2017 May 11. Cell Signal. 2017. PMID: 28502587
-
Eukaryotic elongation factor-2 kinase (eEF2K) signaling in tumor and microenvironment as a novel molecular target.J Mol Med (Berl). 2020 Jun;98(6):775-787. doi: 10.1007/s00109-020-01917-8. Epub 2020 May 7. J Mol Med (Berl). 2020. PMID: 32377852 Review.
-
Regulation and roles of elongation factor 2 kinase.Biochem Soc Trans. 2015 Jun;43(3):328-32. doi: 10.1042/BST20140323. Biochem Soc Trans. 2015. PMID: 26009171 Review.
Cited by
-
Elongation factor-2 kinase is a critical determinant of the fate and antitumor immunity of CD8+ T cells.Sci Adv. 2022 Feb 4;8(5):eabl9783. doi: 10.1126/sciadv.abl9783. Epub 2022 Feb 2. Sci Adv. 2022. PMID: 35108044 Free PMC article.
-
Potential predictive value of plasma heat shock protein 90α in lung cancer.J Int Med Res. 2021 Dec;49(12):3000605211064393. doi: 10.1177/03000605211064393. J Int Med Res. 2021. PMID: 34904468 Free PMC article.
-
Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection.Front Immunol. 2020 Feb 21;11:31. doi: 10.3389/fimmu.2020.00031. eCollection 2020. Front Immunol. 2020. PMID: 32153556 Free PMC article.
-
Proteome Turnover in the Spotlight: Approaches, Applications, and Perspectives.Mol Cell Proteomics. 2021;20:100016. doi: 10.1074/mcp.R120.002190. Epub 2020 Dec 7. Mol Cell Proteomics. 2021. PMID: 33556866 Free PMC article. Review.
-
Integrative proteomics reveals the role of E3 ubiquitin ligase SYVN1 in hepatocellular carcinoma metastasis.Cancer Commun (Lond). 2021 Oct;41(10):1007-1023. doi: 10.1002/cac2.12192. Epub 2021 Jul 1. Cancer Commun (Lond). 2021. PMID: 34196494 Free PMC article.
References
-
- Xie J., Mikolajek H., Pigott C. R., Hooper K. J., Mellows T., Moore C. E., Mohammed H., Werner J. M., Thomas G. J., and Proud C. G. (2015) Molecular mechanism for the control of eukaryotic elongation factor 2 kinase by pH: role in cancer cell survival. Mol. Cell. Biol. 35, 1805–1824 10.1128/MCB.00012-15 - DOI - PMC - PubMed
-
- Leprivier G., Remke M., Rotblat B., Dubuc A., Mateo A. R., Kool M., Agnihotri S., El-Naggar A., Yu B., Somasekharan S. P., Faubert B., Bridon G., Tognon C. E., Mathers J., Thomas R., et al. (2013) The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153, 1064–1079 10.1016/j.cell.2013.04.055 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

