High prognostic value of measurable residual disease detection by flow cytometry in chronic lymphocytic leukemia patients treated with front-line fludarabine, cyclophosphamide, and rituximab, followed by three years of rituximab maintenance
- PMID: 30890600
- PMCID: PMC6821631
- DOI: 10.3324/haematol.2018.204891
High prognostic value of measurable residual disease detection by flow cytometry in chronic lymphocytic leukemia patients treated with front-line fludarabine, cyclophosphamide, and rituximab, followed by three years of rituximab maintenance
Abstract
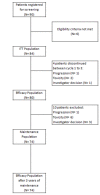
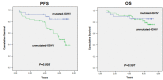
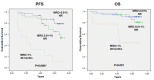
It has been postulated that monitoring measurable residual disease (MRD) could be used as a surrogate marker of progression-free survival (PFS) in chronic lymphocytic leukemia (CLL) patients after treatment with immunochemotherapy regimens. In this study, we analyzed the outcome of 84 patients at 3 years of follow-up after first-line treatment with fludarabine, cyclophosphamide and rituximab (FCR) induction followed by 36 months of rituximab maintenance thearpy. MRD was assessed by a quantitative four-color flow cytometry panel with a sensitivity level of 10-4 Eighty out of 84 evaluable patients (95.2%) achieved at least a partial response or better at the end of induction. After clinical evaluation, 74 patients went into rituximab maintenance and the primary endpoint was assessed in the final analysis at 3 years of follow-up. Bone marrow (BM) MRD analysis was performed after the last planned induction course and every 6 months in cases with detectable residual disease during the 36 months of maintenance therapy. Thirty-seven patients (44%) did not have detectable residual disease in the BM prior to maintenance therapy. Interestingly, 29 patients with detectable residual disease in the BM after induction no longer had detectable disease in the BM following maintenance therapy. After a median followup of 6.30 years, the median overall survival (OS) and PFS had not been reached in patients with either undetectable or detectable residual disease in the BM, who had achieved a complete response at the time of starting maintenance therapy. Interestingly, univariate analysis showed that after rituximab maintenance OS was not affected by IGHV status (mutated vs unmutated OS: 85.7% alive at 7.2 years vs 79.6% alive at 7.3 years, respectively). As per protocol, 15 patients (17.8%), who achieved a complete response and undetectable peripheral blood and BM residual disease after four courses of induction, were allowed to stop fludarabine and cyclophosphamide and complete two additional courses of rituximab and continue with maintenance therapy for 18 cycles. Surprisingly, the outcome in this population was similar to that observed in patients who received the full six cycles of the induction regimen. These data show that, compared to historic controls, patients treated with FCR followed by rituximab maintenance have high-quality responses with fewer relapses and improved OS. The tolerability of this regime is favorable. Furthermore, attaining an early undetectable residual disease status could shorten the duration of chemoimmunotherapy, reducing toxicities and preventing long-term side effects. The analysis of BM MRD after fludarabine-based induction could be a powerful predictor of post-maintenance outcomes in patients with CLL undergoing rituximab maintenance and could be a valuable tool to identify patients at high risk of relapse, influencing further treatment strategies. This trial is registered with EudraCT n. 2007-002733-36 and ClinicalTrials.gov Identifier: NCT00545714.
Copyright© 2019 Ferrata Storti Foundation.
Figures
References
-
- Müller-Hermelink HK, Montserrat E, Catovsky D, et al. Chronic lymphocytic leukaemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp.180–182.
-
- Moreau EJ, Matutes E, A’Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997;108(4):378–382. - PubMed
-
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. - PMC - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. - PubMed

