Yet More Evidence of Collusion: a New Viral Defense System Encoded by Gordonia Phage CarolAnn
- PMID: 30890601
- PMCID: PMC6426606
- DOI: 10.1128/mBio.02417-18
Yet More Evidence of Collusion: a New Viral Defense System Encoded by Gordonia Phage CarolAnn
Abstract
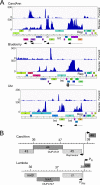
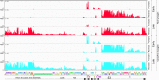
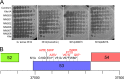
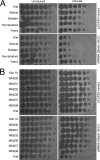
Temperate phages play important roles in the physiology of their bacterial hosts and establish a lysogenic relationship with the host through which prophage-expressed genes confer new phenotypes. A key phenotype is prophage-mediated defense against heterotypic viral attack, in which temperate phages collude with their bacterial host to prevent other phages from attacking, sometimes with exquisite specificity. Such defense systems have been described in Pseudomonas and Mycobacterium phages but are likely widespread throughout the microbial community. Here, we describe a novel prophage-mediated defense system encoded by Gordonia phage CarolAnn, which defends against infection by unrelated phages grouped in cluster CZ. CarolAnn genes 43 and 44 are coexpressed with the repressor and are necessary and sufficient to confer defense against phage Kita and its close relatives. Kita and these relatives are targeted through Kita gene 53, a gene that is of unknown function but which is the location of defense escape mutations that overcome CarolAnn defense. Expression of Kita gene 53 is toxic to Gordonia terrae in the presence of CarolAnn genes 43 and 44, suggesting that defense may be mediated by an abortive infection type of mechanism. CarolAnn genes 43 and 44 are distant relatives of mycobacteriophage Sbash genes 31 and 30, respectively, which also confer viral defense but use a different targeting system.IMPORTANCE Prophage-mediated viral defense systems play a key role in microbial dynamics, as lysogeny is established relatively efficiently, and prophage-expressed genes can strongly inhibit lytic infection of other, unrelated phages. Demonstrating such defense systems in Gordonia terrae suggests that these systems are widespread and that there are a multitude of different systems with different specificities for the attacking phages.
Keywords: Gordonia; bacteriophage; viral defense.
Copyright © 2019 Montgomery et al.
Figures




Similar articles
-
More Evidence of Collusion: a New Prophage-Mediated Viral Defense System Encoded by Mycobacteriophage Sbash.mBio. 2019 Mar 19;10(2):e00196-19. doi: 10.1128/mBio.00196-19. mBio. 2019. PMID: 30890613 Free PMC article.
-
Staphylococcus aureus Prophage-Encoded Protein Causes Abortive Infection and Provides Population Immunity against Kayviruses.mBio. 2023 Apr 25;14(2):e0249022. doi: 10.1128/mbio.02490-22. Epub 2023 Feb 13. mBio. 2023. PMID: 36779718 Free PMC article.
-
Bacteriophage tRNA-dependent lysogeny: requirement of phage-encoded tRNA genes for establishment of lysogeny.mBio. 2024 Feb 14;15(2):e0326023. doi: 10.1128/mbio.03260-23. Epub 2024 Jan 18. mBio. 2024. PMID: 38236026 Free PMC article.
-
The Human Gut Phage Community and Its Implications for Health and Disease.Viruses. 2017 Jun 8;9(6):141. doi: 10.3390/v9060141. Viruses. 2017. PMID: 28594392 Free PMC article. Review.
-
Diverse Antiphage Defenses Are Widespread Among Prophages and Mobile Genetic Elements.Annu Rev Virol. 2024 Sep;11(1):343-362. doi: 10.1146/annurev-virology-100422-125123. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38950439 Review.
Cited by
-
Anti-phage defence through inhibition of virion assembly.Nat Commun. 2024 Feb 22;15(1):1644. doi: 10.1038/s41467-024-45892-x. Nat Commun. 2024. PMID: 38388474 Free PMC article.
-
Mycobacterium Phage Butters-Encoded Proteins Contribute to Host Defense against Viral Attack.mSystems. 2020 Oct 6;5(5):e00534-20. doi: 10.1128/mSystems.00534-20. mSystems. 2020. PMID: 33024050 Free PMC article.
-
The arms race between bacteria and their phage foes.Nature. 2020 Jan;577(7790):327-336. doi: 10.1038/s41586-019-1894-8. Epub 2020 Jan 15. Nature. 2020. PMID: 31942051 Review.
-
The crystal structure of bacteriophage λ RexA provides novel insights into the DNA binding properties of Rex-like phage exclusion proteins.Nucleic Acids Res. 2024 May 8;52(8):4659-4675. doi: 10.1093/nar/gkae212. Nucleic Acids Res. 2024. PMID: 38554102 Free PMC article.
-
Mycobacteriophage Alexphander Gene 94 Encodes an Essential dsDNA-Binding Protein during Lytic Infection.Int J Mol Sci. 2024 Jul 7;25(13):7466. doi: 10.3390/ijms25137466. Int J Mol Sci. 2024. PMID: 39000573 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

