XMAP215 promotes microtubule-F-actin interactions to regulate growth cone microtubules during axon guidance in Xenopuslaevis
- PMID: 30890650
- PMCID: PMC6526707
- DOI: 10.1242/jcs.224311
XMAP215 promotes microtubule-F-actin interactions to regulate growth cone microtubules during axon guidance in Xenopuslaevis
Abstract
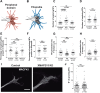
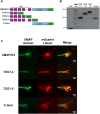
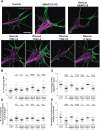
It has long been established that neuronal growth cone navigation depends on changes in microtubule (MT) and F-actin architecture downstream of guidance cues. However, the mechanisms by which MTs and F-actin are dually coordinated remain a fundamentally unresolved question. Here, we report that the well-characterized MT polymerase, XMAP215 (also known as CKAP5), plays an important role in mediating MT-F-actin interaction within the growth cone. We demonstrate that XMAP215 regulates MT-F-actin alignment through its N-terminal TOG 1-5 domains. Additionally, we show that XMAP215 directly binds to F-actin in vitro and co-localizes with F-actin in the growth cone periphery. We also find that XMAP215 is required for regulation of growth cone morphology and response to the guidance cue, Ephrin A5. Our findings provide the first strong evidence that XMAP215 coordinates MT and F-actin interaction in vivo We suggest a model in which XMAP215 regulates MT extension along F-actin bundles into the growth cone periphery and that these interactions may be important to control cytoskeletal dynamics downstream of guidance cues. This article has an associated First Person interview with the first author of the paper.
Keywords: +TIP; CKAP5; Cytoskeleton; F-actin alignment; Growth cones; Plus-end tracking proteins; Super resolution.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Alves-Silva J., Sánchez-Soriano N., Beaven R., Klein M., Parkin J., Millard T. H., Bellen H. J., Venken K. J. T., Ballestrem C. and Kammerer R. A. (2012). Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins). J. Neurosci. 32, 9143-9158. 10.1523/JNEUROSCI.0416-12.2012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

