A MALDI-TOF-based Method for Studying the Transport of BBB Shuttles-Enhancing Sensitivity and Versatility of Cell-Based In Vitro Transport Models
- PMID: 30890722
- PMCID: PMC6424956
- DOI: 10.1038/s41598-019-40973-0
A MALDI-TOF-based Method for Studying the Transport of BBB Shuttles-Enhancing Sensitivity and Versatility of Cell-Based In Vitro Transport Models
Abstract
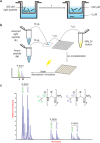
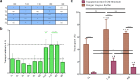
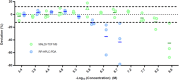
In recent decades, peptide blood-brain barrier shuttles have emerged as a promising solution for brain drugs that are not able to enter this organ. The research and development of these compounds involve the use of in vitro cell-based models of the BBB. Nevertheless, peptide transport quantification implies the use of large amounts of peptide (upper micromolar range for RP-HPLC-PDA) or of derivatives (e.g. fluorophore or quantum-dot attachment, radiolabeling) in the donor compartment in order to enhance the detection of these molecules in the acceptor well, although their structure is highly modified. Therefore, these methodologies either hamper the use of low peptide concentrations, thus hindering mechanistic studies, or do not allow the use of the unmodified peptide. Here we successfully applied a MALDI-TOF MS methodology for transport quantification in an in vitro BBB cell-based model. A light version of the acetylated peptide was evaluated, and the transport was subsequently quantified using a heavy internal standard (isotopically acetylated). We propose that this MALDI-TOF MS approach could also be applied to study the transport across other biological barriers using the appropriate in vitro transport models (e.g. Caco-2, PAMPA).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Chemically synthesized peptide libraries as a new source of BBB shuttles. Use of mass spectrometry for peptide identification.J Pept Sci. 2016 Sep;22(9):577-91. doi: 10.1002/psc.2900. Epub 2016 Jul 20. J Pept Sci. 2016. PMID: 27440580
-
Matrix-assisted laser desorption/ionization imaging mass spectrometry of intraperitoneally injected danegaptide (ZP1609) for treatment of stroke-reperfusion injury in mice.Rapid Commun Mass Spectrom. 2018 Jun 30;32(12):951-958. doi: 10.1002/rcm.8115. Rapid Commun Mass Spectrom. 2018. PMID: 29575411
-
Jumping Hurdles: Peptides Able To Overcome Biological Barriers.Acc Chem Res. 2017 Aug 15;50(8):1847-1854. doi: 10.1021/acs.accounts.7b00204. Epub 2017 Jul 17. Acc Chem Res. 2017. PMID: 28715199
-
The MALDI-TOF mass spectrometric view of the plasma proteome and peptidome.Clin Chem. 2006 Jul;52(7):1223-37. doi: 10.1373/clinchem.2006.069252. Epub 2006 Apr 27. Clin Chem. 2006. PMID: 16644871 Review.
-
Recent advances in the brain-to-blood efflux transport across the blood-brain barrier.Int J Pharm. 2002 Nov 6;248(1-2):15-29. doi: 10.1016/s0378-5173(02)00457-x. Int J Pharm. 2002. PMID: 12429456 Review.
Cited by
-
Molecular determinants for brain targeting by peptides: a meta-analysis approach with experimental validation.Fluids Barriers CNS. 2024 May 27;21(1):45. doi: 10.1186/s12987-024-00545-5. Fluids Barriers CNS. 2024. PMID: 38802930 Free PMC article.
-
Cutting-edge advances in modeling the blood-brain barrier and tools for its reversible permeabilization for enhanced drug delivery into the brain.Cell Biosci. 2023 Jul 27;13(1):137. doi: 10.1186/s13578-023-01079-3. Cell Biosci. 2023. PMID: 37501215 Free PMC article. Review.
-
Antimicrobial cyclic peptides effectively inhibit multiple forms of Borrelia and cross the blood-brain barrier model.Sci Rep. 2025 Feb 20;15(1):6147. doi: 10.1038/s41598-025-90605-z. Sci Rep. 2025. PMID: 39979461 Free PMC article.

