The acidic protein rich in leucines Anp32b is an immunomodulator of inflammation in mice
- PMID: 30890743
- PMCID: PMC6424966
- DOI: 10.1038/s41598-019-41269-z
The acidic protein rich in leucines Anp32b is an immunomodulator of inflammation in mice
Abstract
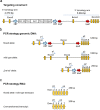
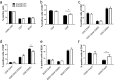
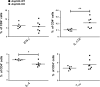
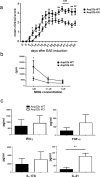
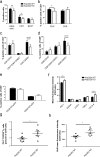
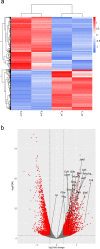
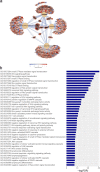
ANP32B belongs to a family of evolutionary conserved acidic nuclear phosphoproteins (ANP32A-H). Family members have been described as multifunctional regulatory proteins and proto-oncogenic factors affecting embryonic development, cell proliferation, apoptosis, and gene expression at various levels. Involvement of ANP32B in multiple processes of cellular life is reflected by the previous finding that systemic gene knockout (KO) of Anp32b leads to embryonic lethality in mice. Here, we demonstrate that a conditional KO of Anp32b is well tolerated in adult animals. However, after immune activation splenocytes isolated from Anp32b KO mice showed a strong commitment towards Th17 immune responses. Therefore, we further analyzed the respective animals in vivo using an experimental autoimmune encephalomyelitis (EAE) model. Interestingly, an exacerbated clinical score was observed in the Anp32b KO mice. This was accompanied by the finding that animal-derived T lymphocytes were in a more activated state, and RNA sequencing analyses revealed hyperactivation of several T lymphocyte-associated immune modulatory pathways, attended by significant upregulation of Tfh cell numbers that altogether might explain the observed strong autoreactive processes. Therefore, Anp32b appears to fulfill a role in regulating adequate adaptive immune responses and, hence, may be involved in dysregulation of pathways leading to autoimmune disorders and/or immune deficiencies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

