Glutathione-related substances maintain cardiomyocyte contractile function in hypoxic conditions
- PMID: 30890744
- PMCID: PMC6425009
- DOI: 10.1038/s41598-019-41266-2
Glutathione-related substances maintain cardiomyocyte contractile function in hypoxic conditions
Abstract
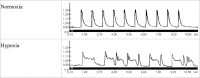
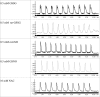
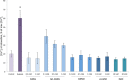
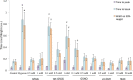
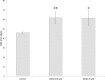
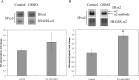
Severe hypoxia leads to decline in cardiac contractility and induces arrhythmic events in part due to oxidative damage to cardiomyocyte proteins including ion transporters. This results in compromised handling of Ca2+ ions that trigger heart contractile machinery. Here, we demonstrate that thiol-containing compounds such as N-acetylcysteine (NAC), glutathione ethyl ester (et-GSH), oxidized tetraethylglutathione (tet-GSSG), oxidized glutathione (GSSG) and S-nitrosoglutathione (GSNO) are capable of reducing negative effects of hypoxia on isolated rat cardiomyocytes. Preincubation of cardiomyocytes with 0.1 mM GSNO, 0.5 mM et-GSH, GSSG, tet-GSSG or with 10 mM NAC allows cells 5-times longer tolerate the hypoxic conditions and elicit regular Ca2+ transients in response to electric pacing. The shape of Ca2+ transients generated in the presence of GSNO, et-GSH and NAC was similar to that observed in normoxic control cardiomyocytes. The leader compound, GSNO, accelerated by 34% the recovery of normal contractile function of isolated rat heart subjected to ischemia-reperfusion. GSNO increased glutathionylation of Na,K-ATPase alpha-2 subunit, the principal ion-transporter of cardiac myocyte sarcolemma, which prevents irreversible oxidation of Na,K-ATPase and regulates its function to support normal Ca2+ ion handling in hypoxic cardiomyocytes. Altogether, GSNO appears effective cardioprotector in hypoxic conditions worth further studies toward its cardiovascular application.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
[The ability of cells to adjust to the low oxigen content associated with Na,K-ATPase glutationilation].Mol Biol (Mosk). 2015 Jan-Feb;49(1):175-83. Mol Biol (Mosk). 2015. PMID: 25916122 Russian.
-
S-nitrosoglutathione/glutathione disulphide/Cu2+-dependent stimulation of L-arginine transport in human platelets.Thromb Res. 1998 Aug 1;91(3):113-20. doi: 10.1016/s0049-3848(98)00060-7. Thromb Res. 1998. PMID: 9733154
-
The mechanism of cardioprotection by S-nitrosoglutathione monoethyl ester in rat isolated heart during cardioplegic ischaemic arrest.Br J Pharmacol. 1996 Oct;119(3):511-8. doi: 10.1111/j.1476-5381.1996.tb15701.x. Br J Pharmacol. 1996. PMID: 8894171 Free PMC article.
-
Glutathionylation of proteins by glutathione disulfide S-oxide.Biochem Pharmacol. 2002 Sep;64(5-6):1049-56. doi: 10.1016/s0006-2952(02)01175-9. Biochem Pharmacol. 2002. PMID: 12213604 Review.
-
The redox pathway of S-nitrosoglutathione, glutathione and nitric oxide in cell to neuron communications.Free Radic Res. 1999 Dec;31(6):641-50. doi: 10.1080/10715769900301211. Free Radic Res. 1999. PMID: 10630687 Review.
Cited by
-
Role of Glutaredoxin-1 and Glutathionylation in Cardiovascular Diseases.Int J Mol Sci. 2020 Sep 16;21(18):6803. doi: 10.3390/ijms21186803. Int J Mol Sci. 2020. PMID: 32948023 Free PMC article. Review.
-
Metabolic Remodeling during Early Cardiac Lineage Specification of Pluripotent Stem Cells.Metabolites. 2023 Oct 17;13(10):1086. doi: 10.3390/metabo13101086. Metabolites. 2023. PMID: 37887411 Free PMC article.
-
Ischemia Enhances the Acute Stretch-Induced Increase in Calcium Spark Rate in Ventricular Myocytes.Front Physiol. 2020 Apr 16;11:289. doi: 10.3389/fphys.2020.00289. eCollection 2020. Front Physiol. 2020. PMID: 32372969 Free PMC article.
References
-
- Vanek T, et al. Heart arrest and myocardial protection by four types of cardioplegic solutions. Exp. Clin. Cardiol. 2014;20:3129–3138.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

