Pathogenic mechanisms of neurodegeneration based on the phenotypic expression of progressive forms of immune-mediated neurologic disease
- PMID: 30890887
- PMCID: PMC6065584
- DOI: 10.2147/DNND.S38353
Pathogenic mechanisms of neurodegeneration based on the phenotypic expression of progressive forms of immune-mediated neurologic disease
Abstract
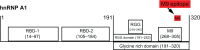
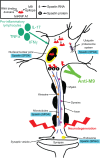
Considering there are no treatments for progressive forms of multiple sclerosis (MS), a comprehensive understanding of the role of neurodegeneration in the pathogenesis of MS should lead to novel therapeutic strategies to treat it. Many studies have implicated viral triggers as a cause of MS, yet no single virus has been exclusively shown to cause MS. Given this, human and animal viral models of MS are used to study its pathogenesis. One example is human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Importantly, HAM/TSP is similar clinically, pathologically, and immunologically to progressive MS. Interestingly, both MS and HAM/TSP patients were found to make antibodies to heterogeneous nuclear ribonucleoprotein (hnRNP) A1, an RNA-binding protein overexpressed in neurons. Anti-hnRNP A1 antibodies reduced neuronal firing and caused neurodegeneration in neuronal cell lines, suggesting the autoantibodies are pathogenic. Further, microarray analyses of neurons exposed to anti-hnRNP A1 antibodies revealed novel pathways of neurodegeneration related to alterations of RNA levels of the spinal paraplegia genes (SPGs). Mutations in SPGs cause hereditary spastic paraparesis, genetic disorders clinically indistinguishable from progressive MS and HAM/TSP. Thus, there is a strong association between involvement of SPGs in neurodegeneration and the clinical phenotype of progressive MS and HAM/TSP patients, who commonly develop spastic paraparesis. Taken together, these data begin to clarify mechanisms of neurodegeneration related to the clinical presentation of patients with chronic immune-mediated neurological disease of the central nervous system, which will give insights into the design of novel therapies to treat these neurological diseases.
Keywords: RNA-binding protein; autoimmunity; heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1); human T-lymphotropic virus type 1 (HTLV-1); multiple sclerosis; neurodegeneration; spastic paraparesis.
Conflict of interest statement
Disclosure Drs Michael Levin and Sangmin Lee have a patent pending titled “Biomarker for neurodegeneration in neurological disease.” All other authors report no conflicts of interest in this paper.
Figures


References
-
- Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 Suppl 3):S22–S31. discussion S43–S54. - PubMed
-
- Peterson JW, Trapp BD. Neuropathobiology of multiple sclerosis. Neurol Clin. 2005;23(1):107–129. vi–vii. - PubMed
-
- Noseworthy J, Lucchinetti C, Rodgriguez M, Weinshenker B. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. - PubMed
-
- Filippi M, Rovaris M, Rocca MA. Imaging primary progressive multiple sclerosis: the contribution of structural, metabolic, and functional MRI techniques. Mult Scler. Jun. 2004;10(Suppl 1):S36–S44. discussion S44–S45. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

