Pathological Tau From Alzheimer's Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo
- PMID: 30890929
- PMCID: PMC6411797
- DOI: 10.3389/fnagi.2019.00034
Pathological Tau From Alzheimer's Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo
Abstract
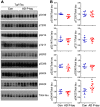
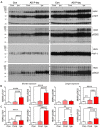
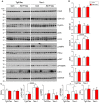
Neurofibrillary tangles (NFTs) made up of hyperphosphorylated tau are a histopathological hallmark of Alzheimer's disease (AD) and related tauopathies. Hyperphosphorylation of tau is responsible for its loss of normal physiological function, gain of toxicity and its aggregation to form NFTs. Injection of misfolded tau seeds into mouse brain induces tau aggregation, but the nature of tau phosphorylation in pathologic tau seeded pathology is unclear. In the present study, we injected hyperphosphorylated and oligomeric tau isolated from AD brain (AD P-tau) into hippocampus of human tau transgenic mice and found that in addition to tau aggregation/pathology, tau was hyperphosphorylated at Ser202/Thr205, Thr212, Ser214, Thr217, Ser262, and Ser422 in AD P-tau injected hippocampus and at Ser422 in the contralateral hippocampus and in the ipsilateral cortex. AD P-tau-induced AD-like high molecular weight aggregation of tau that was SDS- and reducing agent-resistant and site-specifically hyperphosphorylated in the ipsilateral hippocampus. There were no detectable alterations in levels of tau phosphatases or tau kinases in AD P-tau-injected brains. Furthermore, we found that hyperphosphorylated tau was easier to be captured by AD P-tau and that aggregated tau was more difficult to be dephosphorylated than the non-aggregated tau by protein phosphatase 2A (PP2A). Based on these findings, we speculate that AD P-tau seeds hyperphosphorylated tau to form aggregates, which resist to the dephosphorylation by PP2A, resulting in hyperphosphorylation and pathology of tau.
Keywords: AD P-tau; Alzheimer’s disease; hyperphosphorylation of tau; propagation of tau pathology; tau pathology.
Figures





References
-
- Ahmed Z., Cooper J., Murray T. K., Garn K., McNaughton E., Clarke H., et al. . (2014). A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683. 10.1007/s00401-014-1254-6 - DOI - PMC - PubMed
-
- Alonso A. D., Grundke-Iqbal I., Iqbal K. (1996). Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 2, 783–787. - PubMed
-
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. (1992). Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

