Impaired Contracture of 3D Collagen Constructs by Fibronectin-Deficient Murine Fibroblasts
- PMID: 30890950
- PMCID: PMC6413635
- DOI: 10.3389/fphys.2019.00166
Impaired Contracture of 3D Collagen Constructs by Fibronectin-Deficient Murine Fibroblasts
Abstract
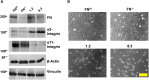
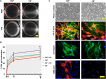
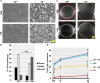
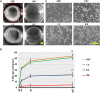
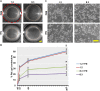
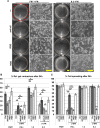
Fibronectin (FN) is an extracellular matrix glycoprotein that is abundantly expressed by fibroblasts in contracting wounds, where it mediates cell adhesion, migration and proliferation. FN also efficiently binds to collagen. Therefore, we and others hypothesized that FN and its cellular receptor integrin α5β1 might be involved in collagen matrix contracture by acting as linkers. However, there are conflicting reports on this issue. Moreover, several publications suggest an important role of collagen-binding integrin receptors α2β1 and α11β1 in collagen matrix contracture. Therefore, the aim of the present study was to determine the contributions of FN-integrin α5β1 interactions relative to those of collagen receptors α2β1 and α11β1 in this process. To assess the role of cellular FN directly, we employed FN-deficient mouse fibroblasts, subjected them to a collagen gel contracture assay in vitro, and compared them to their wildtype counterparts. Exogenous FN was removed from serum-containing medium. For dissecting the role of major collagen receptors, we used two FN-deficient mouse fibroblast lines that both possess integrin α5β1 but differ in their collagen-binding integrins. Embryo-derived FN-null fibroblasts, which express α11- but no α2-integrin, barely spread and tended to cluster on collagen gels. Moreover, FN-null fibroblasts required exogenously added FN to assemble α5β1-integrin in fibrillar adhesion contacts, and to contract collagen matrices. In contrast, postnatal kidney fibroblasts were found to express α2- but barely α11-integrin. When FN expression was suppressed in these cells by shRNA transfection, they were able to spread on and partially contract collagen gels in the absence of exogenous FN. Also in this case, however, collagen contracture was stimulated by adding FN to the medium. Antibody to integrin α5β1 or RGD peptide completely abolished collagen contracture by FN-deficient fibroblasts stimulated by FN addition. We conclude that although collagen-binding integrins (especially α2β1) can mediate contracture of fibrillar collagen gels by murine fibroblasts to some extent, full activity is causally linked to the presence of pericellular FN and its receptor integrin α5β1.
Keywords: alpha5-integrin; collagen; collagen contraction; collagen contracture; fibroblasts; fibronectin.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

