A Bayesian Account of the Sensory-Motor Interactions Underlying Symptoms of Tourette Syndrome
- PMID: 30890965
- PMCID: PMC6412155
- DOI: 10.3389/fpsyt.2019.00029
A Bayesian Account of the Sensory-Motor Interactions Underlying Symptoms of Tourette Syndrome
Abstract
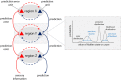
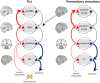
Tourette syndrome is a hyperkinetic movement disorder. Characteristic features include tics, recurrent movements that are experienced as compulsive and "unwilled"; uncomfortable premonitory sensations that resolve through tic release; and often, the ability to suppress tics temporarily. We demonstrate how these symptoms and features can be understood in terms of aberrant predictive (Bayesian) processing in hierarchical neural systems, explaining specifically: why tics arise, their "unvoluntary" nature, how premonitory sensations emerge, and why tic suppression works-sometimes. In our model, premonitory sensations and tics are generated through over-precise priors for sensation and action within somatomotor regions of the striatum. Abnormally high precision of priors arises through the dysfunctional synaptic integration of cortical inputs. These priors for sensation and action are projected into primary sensory and motor areas, triggering premonitory sensations and tics, which in turn elicit prediction errors for unexpected feelings and movements. We propose experimental paradigms to validate this Bayesian account of tics. Our model integrates behavioural, neuroimaging, and computational approaches to provide mechanistic insight into the pathophysiological basis of Tourette syndrome.
Keywords: Tourette syndrome; active inference; basal ganglia; insula; motor cortex; tics.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

