Isolated Silymarin Flavonoids Increase Systemic and Hepatic Bilirubin Concentrations and Lower Lipoperoxidation in Mice
- PMID: 30891115
- PMCID: PMC6390243
- DOI: 10.1155/2019/6026902
Isolated Silymarin Flavonoids Increase Systemic and Hepatic Bilirubin Concentrations and Lower Lipoperoxidation in Mice
Abstract
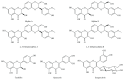
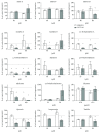
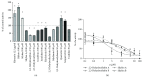
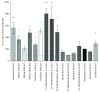
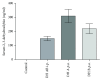
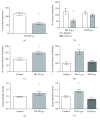
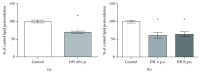
Bilirubin is considered to be one of the most potent endogenous antioxidants in humans. Its serum concentrations are predominantly affected by the activity of hepatic bilirubin UDP-glucuronosyl transferase (UGT1A1). Our objective was to analyze the potential bilirubin-modulating effects of natural polyphenols from milk thistle (Silybum marianum), a hepatoprotective herb. Human hepatoblastoma HepG2 cells were exposed to major polyphenolic compounds isolated from milk thistle. Based on in vitro studies, 2,3-dehydrosilybins A and B were selected as the most efficient compounds and applied either intraperitoneally or orally for seven days to C57BL/6 mice. After, UGT1A1 mRNA expression, serum, intrahepatic bilirubin concentrations, and lipoperoxidation in the liver tissue were analyzed. All natural polyphenols used increased intracellular concentration of bilirubin in HepG2 cells to a similar extent as atazanavir, a known bilirubinemia-enhancing agent. Intraperitoneal application of 2,3-dehydrosilybins A and B (the most efficient flavonoids from in vitro studies) to mice (50 mg/kg) led to a significant downregulation of UGT1A1 mRNA expression (46 ± 3% of controls, p < 0.005) in the liver and also to a significant increase of the intracellular bilirubin concentration (0.98 ± 0.03vs.1.21 ± 0.02 nmol/mg, p < 0.05). Simultaneously, a significant decrease of lipoperoxidation (61 ± 2% of controls, p < 0.005) was detected in the liver tissue of treated animals, and similar results were also observed after oral treatment. Importantly, both application routes also led to a significant elevation of serum bilirubin concentrations (125 ± 3% and 160 ± 22% of the controls after intraperitoneal and oral administration, respectively, p < 0.005 in both cases). In conclusion, polyphenolic compounds contained in silymarin, in particular 2,3-dehydrosilybins A and B, affect hepatic and serum bilirubin concentrations, as well as lipoperoxidation in the liver. This phenomenon might contribute to the hepatoprotective effects of silymarin.
Figures
Similar articles
-
Drug-drug interactions of silymarin on the perspective of pharmacokinetics.J Ethnopharmacol. 2009 Jan 21;121(2):185-93. doi: 10.1016/j.jep.2008.10.036. Epub 2008 Nov 8. J Ethnopharmacol. 2009. PMID: 19041708 Review.
-
Effects of flavonoids on the resistance of microsomes to lipid peroxidation in vitro and ex vivo.Bull Exp Biol Med. 2003 Dec;136(6):572-5. doi: 10.1023/b:bebm.0000020207.67705.59. Bull Exp Biol Med. 2003. PMID: 15500075
-
Effect of flavonoids from Prosthechea michuacana on carbon tetrachloride induced acute hepatotoxicity in mice.Pharm Biol. 2011 Nov;49(11):1121-7. doi: 10.3109/13880209.2011.570766. Pharm Biol. 2011. PMID: 22014261
-
Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in the human hepatoma cell line hep G2.Drug Metab Dispos. 2000 Sep;28(9):1077-82. Drug Metab Dispos. 2000. PMID: 10950852
-
Advances in pharmacological studies of silymarin.Mem Inst Oswaldo Cruz. 1991;86 Suppl 2:79-85. doi: 10.1590/s0074-02761991000600020. Mem Inst Oswaldo Cruz. 1991. PMID: 1842018 Review.
Cited by
-
Role of Natural Compounds Modulating Heme Catabolic Pathway in Gut, Liver, Cardiovascular, and Brain Diseases.Biomolecules. 2024 Jan 2;14(1):63. doi: 10.3390/biom14010063. Biomolecules. 2024. PMID: 38254662 Free PMC article. Review.
-
Therapeutic effects of mesenchymal stem cells-conditioned medium derived from suspension cultivation or silymarin on liver failure mice.Mol Biol Rep. 2022 Nov;49(11):10315-10325. doi: 10.1007/s11033-022-07785-4. Epub 2022 Sep 12. Mol Biol Rep. 2022. PMID: 36097106
-
Metabolism of 2,3-Dehydrosilybin A and 2,3-Dehydrosilybin B: A Study with Human Hepatocytes and Recombinant UDP-Glucuronosyltransferases and Sulfotransferases.Antioxidants (Basel). 2021 Jun 14;10(6):954. doi: 10.3390/antiox10060954. Antioxidants (Basel). 2021. PMID: 34198653 Free PMC article.
-
Natural Product Heme Oxygenase Inducers as Treatment for Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2020 Dec 14;21(24):9493. doi: 10.3390/ijms21249493. Int J Mol Sci. 2020. PMID: 33327438 Free PMC article. Review.
-
Poor chemical and microbiological quality of the commercial milk thistle-based dietary supplements may account for their reported unsatisfactory and non-reproducible clinical outcomes.Sci Rep. 2019 Jul 31;9(1):11118. doi: 10.1038/s41598-019-47250-0. Sci Rep. 2019. PMID: 31366891 Free PMC article.
References
-
- Molzer C., Huber H., Steyrer A., et al. Interaction between TNFone and tetrapyrroles may account for their anti-genotoxic effects — a novel mechanism for DNA-protection. Journal of Porphyrins and Phthalocyanines. 2013;17(12):1157–1166. doi: 10.1142/S1088424613500995. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

