Di-aryl Sulfonamide Motif Adds π-Stacking Bulk in Negative Allosteric Modulators of the NMDA Receptor
- PMID: 30891121
- PMCID: PMC6421532
- DOI: 10.1021/acsmedchemlett.8b00395
Di-aryl Sulfonamide Motif Adds π-Stacking Bulk in Negative Allosteric Modulators of the NMDA Receptor
Abstract
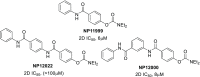
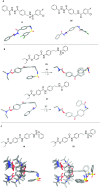
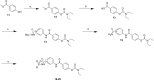
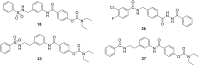
The N-methyl-d-aspartate receptor plays a critical role in central nervous system processes. Its diverse properties, as well as hypothesized role in neurological disease, render NMDA receptors a target of interest for the development of therapeutically relevant modulators. A number of subunit-selective modulators have been reported in the literature, one of which is TCN-201, a GluN2A-selective negative allosteric modulator. Recently, it was determined from a cocrystallization study of TCN-201 with the NMDA receptor that a unique active pose exists in which the sulfonamide group of TCN-201 incorporates a π-π stacking interaction between the two adjacent aryl rings that allows it to make important contacts with the protein. This finding led us to investigate whether this unique structural feature of the diaryl sulfonamide could be incorporated into other modulators that act on distinct pockets. To test whether this idea might have more general utility, we added an aryl ring plus the sulfonamide linker modification to a previously published series of GluN2C- and GluN2D-selective negative allosteric modulators that bind to an entirely different pocket. Herein, we report data suggesting that this structural modification of the NAB-14 series of modulators was tolerated and, in some instances, enhanced potency. These results suggest that this motif may be a reliable means for introducing a π-π stacking element to molecular scaffolds that could improve activity if it allowed access to ligand-protein interactions not accessible from one planar aromatic group.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.C.L., S.F.T., S.L.S., and D.S.M. are coinventors on Emory-owned intellectual property associated with allosteric modulators of NMDA receptor function. S.F.T. is a paid consultant for Janssen, the principal investigator on a research grant from Janssen to Emory University, a member of the SAB for Sage Therapeutics, recipient of royalties from licensing software, and a cofounder of NeurOp Inc. D.C.L. is a member of the BOD for NeurOp Inc.
Figures

Similar articles
-
The Bioactive Protein-Ligand Conformation of GluN2C-Selective Positive Allosteric Modulators Bound to the NMDA Receptor.Mol Pharmacol. 2018 Feb;93(2):141-156. doi: 10.1124/mol.117.110940. Epub 2017 Dec 14. Mol Pharmacol. 2018. PMID: 29242355 Free PMC article.
-
Systematic variation of the benzoylhydrazine moiety of the GluN2A selective NMDA receptor antagonist TCN-201.Eur J Med Chem. 2018 Oct 5;158:259-269. doi: 10.1016/j.ejmech.2018.09.006. Epub 2018 Sep 6. Eur J Med Chem. 2018. PMID: 30218911
-
Chimeric Glutamate Receptor Subunits Reveal the Transmembrane Domain Is Sufficient for NMDA Receptor Pore Properties but Some Positive Allosteric Modulators Require Additional Domains.J Neurosci. 2016 Aug 24;36(34):8815-25. doi: 10.1523/JNEUROSCI.0345-16.2016. J Neurosci. 2016. PMID: 27559165 Free PMC article.
-
Recent progress in allosteric modulators for GluN2A subunit and development of GluN2A-selective nuclear imaging probes.J Labelled Comp Radiopharm. 2019 Jun 30;62(8):552-560. doi: 10.1002/jlcr.3744. J Labelled Comp Radiopharm. 2019. PMID: 31037756 Review.
-
NMDA receptor modulators: an updated patent review (2013-2014).Expert Opin Ther Pat. 2014 Dec;24(12):1349-66. doi: 10.1517/13543776.2014.972938. Epub 2014 Oct 29. Expert Opin Ther Pat. 2014. PMID: 25351527 Free PMC article. Review.
Cited by
-
Progresses in GluN2A-containing NMDA Receptors and their Selective Regulators.Cell Mol Neurobiol. 2023 Jan;43(1):139-153. doi: 10.1007/s10571-021-01185-1. Epub 2022 Jan 3. Cell Mol Neurobiol. 2023. PMID: 34978648 Free PMC article. Review.
References
-
- Collingridge G. L.; Volianskis A.; Bannister N.; France G.; Hanna L.; Mercier M.; Tidball P.; Fang G.; Irvine M. W.; Costa B. M.; Monaghan D. T.; Bortolotto Z. A.; Molnár E.; Lodge D.; Jane D. E. The NMDA Receptor as a Target for Cognitive Enhancement. Neuropharmacology 2013, 64, 13–26. 10.1016/j.neuropharm.2012.06.051. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

