Use of a Conformational-Switching Mechanism to Modulate Exposed Polarity: Discovery of CCR2 Antagonist BMS-741672
- PMID: 30891130
- PMCID: PMC6421546
- DOI: 10.1021/acsmedchemlett.8b00439
Use of a Conformational-Switching Mechanism to Modulate Exposed Polarity: Discovery of CCR2 Antagonist BMS-741672
Abstract
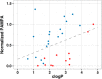
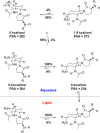
We encountered a dilemma in the course of studying a series of antagonists of the G-protein coupled receptor CC chemokine receptor-2 (CCR2): compounds with polar C3 side chains exhibited good ion channel selectivity but poor oral bioavailability, whereas compounds with lipophilic C3 side chains exhibited good oral bioavailability in preclinical species but poor ion channel selectivity. Attempts to solve this through the direct modulation of physicochemical properties failed. However, the installation of a protonation-dependent conformational switching mechanism resolved the problem because it enabled a highly selective and relatively polar molecule to access a small population of a conformer with lower polar surface area and higher membrane permeability. Optimization of the overall properties in this series yielded the CCR2 antagonist BMS-741672 (7), which embodied properties suitable for study in human clinical trials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Discovery of BMS-753426: A Potent Orally Bioavailable Antagonist of CC Chemokine Receptor 2.ACS Med Chem Lett. 2021 May 25;12(6):969-975. doi: 10.1021/acsmedchemlett.1c00082. eCollection 2021 Jun 10. ACS Med Chem Lett. 2021. PMID: 34141082 Free PMC article.
-
Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists.Nature. 2016 Dec 15;540(7633):458-461. doi: 10.1038/nature20605. Epub 2016 Dec 7. Nature. 2016. PMID: 27926736 Free PMC article.
-
The discovery of BMS-457, a potent and selective CCR1 antagonist.Bioorg Med Chem Lett. 2013 Jul 1;23(13):3833-40. doi: 10.1016/j.bmcl.2013.04.079. Epub 2013 May 7. Bioorg Med Chem Lett. 2013. PMID: 23707259
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Progress in the discovery of CC chemokine receptor 2 antagonists, 2009 - 2012.Expert Opin Ther Pat. 2013 May;23(5):549-68. doi: 10.1517/13543776.2013.771168. Epub 2013 Feb 22. Expert Opin Ther Pat. 2013. PMID: 23428142 Review.
Cited by
-
Molecular chameleons in drug discovery.Nat Rev Chem. 2024 Jan;8(1):45-60. doi: 10.1038/s41570-023-00563-1. Epub 2023 Dec 20. Nat Rev Chem. 2024. PMID: 38123688 Review.
-
Molecular determinants of antagonist interactions with chemokine receptors CCR2 and CCR5.bioRxiv [Preprint]. 2024 Feb 12:2023.11.15.567150. doi: 10.1101/2023.11.15.567150. bioRxiv. 2024. PMID: 38014122 Free PMC article. Preprint.
-
Role of chemokine systems in cancer and inflammatory diseases.MedComm (2020). 2022 Jun 8;3(2):e147. doi: 10.1002/mco2.147. eCollection 2022 Jun. MedComm (2020). 2022. PMID: 35702353 Free PMC article. Review.
-
Discovery of BMS-753426: A Potent Orally Bioavailable Antagonist of CC Chemokine Receptor 2.ACS Med Chem Lett. 2021 May 25;12(6):969-975. doi: 10.1021/acsmedchemlett.1c00082. eCollection 2021 Jun 10. ACS Med Chem Lett. 2021. PMID: 34141082 Free PMC article.
-
Macrocycles in Drug Discovery─Learning from the Past for the Future.J Med Chem. 2023 Apr 27;66(8):5377-5396. doi: 10.1021/acs.jmedchem.3c00134. Epub 2023 Apr 5. J Med Chem. 2023. PMID: 37017513 Free PMC article. Review.
References
-
- Waring M. J.; Arrowsmith J.; Leach A. R.; Leeson P. D.; Mandrell S.; Owen R. M.; Pairaudeau G.; Pennie W. D.; Pickett S. D.; Wang J.; Wallace O.; Weir A. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discovery 2015, 14 (7), 475–486. 10.1038/nrd4609. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

