Complex immune responses and molecular reactions to pathogens and disease in a desert reptile (Gopherus agassizii)
- PMID: 30891197
- PMCID: PMC6405529
- DOI: 10.1002/ece3.4897
Complex immune responses and molecular reactions to pathogens and disease in a desert reptile (Gopherus agassizii)
Abstract
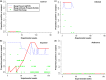
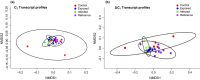
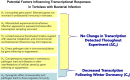
Immune function plays an important role in an animal's defense against infectious disease. In reptiles, immune responses may be complex and counterintuitive, and diagnostic tools used to identify infection, such as induced antibody responses are limited. Recent studies using gene transcription profiling in tortoises have proven useful in identifying immune responses to various intrinsic and extrinsic stressors. As part of a larger experiment with Mojave desert tortoises (Gopherus agassizii), we facilitated the transmission of the pathogenic bacteria, Mycoplasma agassizii (Myag), to naïve adults and measured innate and induced immune reactions over time. Specifically, we evaluated clinical condition, presence of Myag in the nasal/oral cavity, induced antibody responses specific to Myag, and measured molecular reactions (gene transcript profiles) in 15 captive tortoises classified as naïve, exposed, or infected and 14 wild tortoises for comparison. Myag was confirmed inside the nasal/oral cavity in exposed tortoises within 30-60 days of introduction to infected animals, yet we did not detect Myag specific induced antibody responses in these individuals until 420-595 days post exposure. Surprisingly, we found no overall differences in the gene transcript profiles between our experimental treatment groups throughout this study. This work highlights the complexities in assessing immune function and diagnosing pathogen related infections in tortoises and other reptiles.
Keywords: Mycoplasma agassizii; desert tortoise; immunity; mRNA; transcription.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Molecular methods to detect Mycoplasma spp. And Testudinid herpesvirus 2 in desert tortoises (Gopherus agassizii) and implications for disease management.J Wildl Dis. 2014 Oct;50(4):757-66. doi: 10.7589/2013-09-231. Epub 2014 Aug 14. J Wildl Dis. 2014. PMID: 25121400
-
Western blot can distinguish natural and acquired antibodies to Mycoplasma agassizii in the desert tortoise (Gopherus agassizii).J Microbiol Methods. 2008 Dec;75(3):464-71. doi: 10.1016/j.mimet.2008.07.022. Epub 2008 Jul 26. J Microbiol Methods. 2008. PMID: 18708096
-
Coupling gene-based and classic veterinary diagnostics improves interpretation of health and immune function in the Agassiz's desert tortoise (Gopherus agassizii).Conserv Physiol. 2017 Jun 16;5(1):cox037. doi: 10.1093/conphys/cox037. eCollection 2017. Conserv Physiol. 2017. PMID: 28835840 Free PMC article.
-
Mycoplasmosis and upper respiratory tract disease of tortoises: a review and update.Vet J. 2014 Sep;201(3):257-64. doi: 10.1016/j.tvjl.2014.05.039. Epub 2014 Jun 4. Vet J. 2014. PMID: 24951264 Review.
-
Role of urinary and cloacal bladders in chelonian water economy: historical and comparative perspectives.Biol Rev Camb Philos Soc. 1998 Nov;73(4):347-66. doi: 10.1017/s0006323198005210. Biol Rev Camb Philos Soc. 1998. PMID: 9951413 Review.
Cited by
-
Immune and sex-biased gene expression in the threatened Mojave desert tortoise, Gopherus agassizii.PLoS One. 2020 Aug 26;15(8):e0238202. doi: 10.1371/journal.pone.0238202. eCollection 2020. PLoS One. 2020. PMID: 32846428 Free PMC article.
-
Island of misfit tortoises: waif gopher tortoise health assessment following translocation.Conserv Physiol. 2022 Jul 27;10(1):coac051. doi: 10.1093/conphys/coac051. eCollection 2022. Conserv Physiol. 2022. PMID: 37501911 Free PMC article.
-
Meta-Transcriptomic Discovery of a Divergent Circovirus and a Chaphamaparvovirus in Captive Reptiles with Proliferative Respiratory Syndrome.Viruses. 2020 Sep 25;12(10):1073. doi: 10.3390/v12101073. Viruses. 2020. PMID: 32992674 Free PMC article.
-
Tissue tropism, pathology, and pathogenesis of West Nile virus infection in saltwater crocodile (Crocodylus porosus).PLoS Negl Trop Dis. 2025 Aug 4;19(8):e0013385. doi: 10.1371/journal.pntd.0013385. eCollection 2025 Aug. PLoS Negl Trop Dis. 2025. PMID: 40758745 Free PMC article.
References
-
- Adamo, S. A. (2004). How should behavioral ecologists interpret measurements of immunity? Animal Behavior, 68, 1443–1449.
-
- Aiello, C. M. , Esque, T. C. , Nussear, K. E. , Emblidge, P. G. , & Hudson, P. J. (2018). The slow dynamics of mycoplasma infections in a tortoise host reveal heterogeneity pertinent to pathogen transmission and monitoring. Epidemiology and Infection, 147 e12, 1–10. 10.1017/S09502688002613 - DOI - PubMed
-
- Aiello, C. M. , Nussear, K. E. , Esque, T. C. , Emblidge, P. G. , Sah, P. , Sansal, S. , & Hudson, P. J. (2016). Host contact and shedding patterns clarify variation in pathogen exposure and transmission in threatened tortoise Gopherus agassizii: Implications for disease modeling and management. Journal of Animal Ecology, 85(3), 829–842. - PubMed
-
- Alberts, B. , Johnson, A. , Lewis, J. , Raff, M. , Roberts, K. , & Walter, P. (2002). Molecular biology of the cell, 4th ed. New York, NY: Garland Science.
LinkOut - more resources
Full Text Sources
Research Materials

