Layer boundary evolution method for macular OCT layer segmentation
- PMID: 30891330
- PMCID: PMC6420297
- DOI: 10.1364/BOE.10.001064
Layer boundary evolution method for macular OCT layer segmentation
Abstract
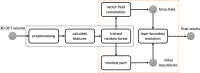
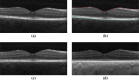
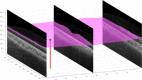
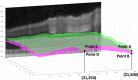
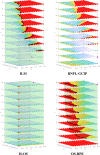
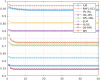
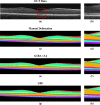
Optical coherence tomography (OCT) is used to produce high resolution depth images of the retina and is now the standard of care for in-vivo ophthalmological assessment. It is also increasingly being used for evaluation of neurological disorders such as multiple sclerosis (MS). Automatic segmentation methods identify the retinal layers of the macular cube providing consistent results without intra- and inter-rater variation and is faster than manual segmentation. In this paper, we propose a fast multi-layer macular OCT segmentation method based on a fast level set method. Our framework uses contours in an optimized approach specifically for OCT layer segmentation over the whole macular cube. Our algorithm takes boundary probability maps from a trained random forest and iteratively refines the prediction to subvoxel precision. Evaluation on both healthy and multiple sclerosis subjects shows that our method is statistically better than a state-of-the-art graph-based method.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures


Similar articles
-
Multi-layer Fast Level Set Segmentation for Macular OCT.Proc IEEE Int Symp Biomed Imaging. 2018 Apr;2018:1445-1448. doi: 10.1109/ISBI.2018.8363844. Epub 2018 May 24. Proc IEEE Int Symp Biomed Imaging. 2018. PMID: 31853331 Free PMC article.
-
Multiple-object geometric deformable model for segmentation of macular OCT.Biomed Opt Express. 2014 Mar 4;5(4):1062-74. doi: 10.1364/BOE.5.001062. eCollection 2014 Apr 1. Biomed Opt Express. 2014. PMID: 24761289 Free PMC article.
-
Retinal layer segmentation of macular OCT images using boundary classification.Biomed Opt Express. 2013 Jun 14;4(7):1133-52. doi: 10.1364/BOE.4.001133. Print 2013 Jul 1. Biomed Opt Express. 2013. PMID: 23847738 Free PMC article.
-
Comparison of point estimates and average thicknesses of retinal layers measured using manual optical coherence tomography segmentation for quantification of retinal neurodegeneration in multiple sclerosis.Curr Eye Res. 2013 Jan;38(1):224-8. doi: 10.3109/02713683.2012.722243. Epub 2012 Sep 6. Curr Eye Res. 2013. PMID: 22954302 Free PMC article.
-
Towards Topological Correct Segmentation of Macular OCT from Cascaded FCNs.Fetal Infant Ophthalmic Med Image Anal (2017). 2017 Sep;10554:202-209. doi: 10.1007/978-3-319-67561-9_23. Epub 2017 Sep 9. Fetal Infant Ophthalmic Med Image Anal (2017). 2017. PMID: 31355372 Free PMC article.
Cited by
-
Deep learning network with differentiable dynamic programming for retina OCT surface segmentation.Biomed Opt Express. 2023 Jun 8;14(7):3190-3202. doi: 10.1364/BOE.492670. eCollection 2023 Jul 1. Biomed Opt Express. 2023. PMID: 37497505 Free PMC article.
-
Evaluating White Matter Lesion Segmentations with Refined Sørensen-Dice Analysis.Sci Rep. 2020 May 19;10(1):8242. doi: 10.1038/s41598-020-64803-w. Sci Rep. 2020. PMID: 32427874 Free PMC article.
-
Artificial intelligence method based on multi-feature fusion for automatic macular edema (ME) classification on spectral-domain optical coherence tomography (SD-OCT) images.Front Neurosci. 2023 Jan 30;17:1097291. doi: 10.3389/fnins.2023.1097291. eCollection 2023. Front Neurosci. 2023. PMID: 36793539 Free PMC article.
-
Structured layer surface segmentation for retina OCT using fully convolutional regression networks.Med Image Anal. 2021 Feb;68:101856. doi: 10.1016/j.media.2020.101856. Epub 2020 Oct 14. Med Image Anal. 2021. PMID: 33260113 Free PMC article.
-
Longitudinal deep network for consistent OCT layer segmentation.Biomed Opt Express. 2023 Apr 3;14(5):1874-1893. doi: 10.1364/BOE.487518. eCollection 2023 May 1. Biomed Opt Express. 2023. PMID: 37206119 Free PMC article.
References
-
- Saidha S., Eckstein C., Ratchford J. N., “Optical coherence tomography as a marker of axonal damage in multiple sclerosis,” Int. J. Clin. Rev. (2010).10.5275/ijcr.2010.10.01 - DOI
-
- Saidha S., Sotirchos E. S., Ibrahim M. A., Crainiceanu C. M., Gelfand J. M., Sepah Y. J., Ratchford J. N., Oh J., Seigo M. A., Newsome S. D., Balcer L. J., Frohman E. M., Green A. J., Nguyen Q. D., Calabresi P. A., “Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study,” The Lancet Neurol. 11, 963–972 (2012).10.1016/S1474-4422(12)70213-2 - DOI - PMC - PubMed
-
- Saidha S., Syc S. B., Durbin M. K., Eckstein C., Oakley J. D., Meyer S. A., Conger A., Frohman T. C., Newsome S., Ratchford J. N., Frohman E. M., Calabresi P. A., “Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness,” Multiple Scler. J. 17, 1449–1463 (2011).10.1177/1352458511418630 - DOI - PubMed
-
- DeBuc D. C., Somfai G. M., “Early detection of retinal thickness changes in diabetes using optical coherence tomography,” Med. Sci. Monit. 16, MT15–MT21 (2010). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
