Identification of a α-helical molten globule intermediate and structural characterization of β-cardiotoxin, an all β-sheet protein isolated from the venom of Ophiophagus hannah (king cobra)
- PMID: 30891862
- PMCID: PMC6459992
- DOI: 10.1002/pro.3605
Identification of a α-helical molten globule intermediate and structural characterization of β-cardiotoxin, an all β-sheet protein isolated from the venom of Ophiophagus hannah (king cobra)
Abstract
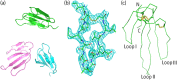
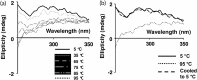
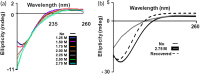
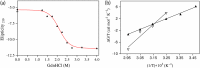
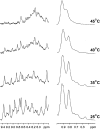
β-Cardiotoxin is a novel member of the snake venom three-finger toxin (3FTX) family. This is the first exogenous protein to antagonize β-adrenergic receptors and thereby causing reduction in heart rates (bradycardia) when administered into animals, unlike the conventional cardiotoxins as reported earlier. 3FTXs are stable all β-sheet peptides with 60-80 amino acid residues. Here, we describe the three-dimensional crystal structure of β-cardiotoxin together with the identification of a molten globule intermediate in the unfolding pathway of this protein. In spite of the overall structural similarity of this protein with conventional cardiotoxins, there are notable differences observed at the loop region and in the charge distribution on the surface, which are known to be critical for cytolytic activity of cardiotoxins. The molten globule intermediate state present in the thermal unfolding pathway of β-cardiotoxin was however not observed during the chemical denaturation of the protein. Interestingly, circular dichroism (CD) and NMR studies revealed the presence of α-helical secondary structure in the molten globule intermediate. These results point to substantial conformational plasticity of β-cardiotoxin, which might aid the protein in responding to the sometimes conflicting demands of structure, stability, and function during its biological lifetime.
Keywords: beta-blocker; molten globule; non-hierarchical protein folding; thermal denaturation and structural transition; three-finger toxin.
© 2019 The Protein Society.
Figures



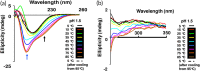
Similar articles
-
Beta-cardiotoxin: a new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity.FASEB J. 2007 Nov;21(13):3685-95. doi: 10.1096/fj.07-8658com. Epub 2007 Jul 6. FASEB J. 2007. PMID: 17616557
-
Identification of 'molten globule'-like state in all beta-sheet protein.Biochem Biophys Res Commun. 1995 Feb 15;207(2):536-43. doi: 10.1006/bbrc.1995.1221. Biochem Biophys Res Commun. 1995. PMID: 7864840
-
Hexafluoroacetone hydrate as a structure modifier in proteins: characterization of a molten globule state of hen egg-white lysozyme.Protein Sci. 1997 May;6(5):1065-73. doi: 10.1002/pro.5560060513. Protein Sci. 1997. PMID: 9144778 Free PMC article.
-
Snake venom cardiotoxins-structure, dynamics, function and folding.J Biomol Struct Dyn. 1997 Dec;15(3):431-63. doi: 10.1080/07391102.1997.10508957. J Biomol Struct Dyn. 1997. PMID: 9439993 Review.
-
The molten globule state of alpha-lactalbumin.FASEB J. 1996 Jan;10(1):102-9. doi: 10.1096/fasebj.10.1.8566530. FASEB J. 1996. PMID: 8566530 Review.
Cited by
-
Cardiovascular Effects of Snake Toxins: Cardiotoxicity and Cardioprotection.Acta Naturae. 2021 Jul-Sep;13(3):4-14. doi: 10.32607/actanaturae.11375. Acta Naturae. 2021. PMID: 34707893 Free PMC article.
-
Suppression of cardiomyocyte functions by β-CTX isolated from the Thai king cobra (Ophiophagus hannah) venom via an alternative method.J Venom Anim Toxins Incl Trop Dis. 2020 Jul 17;26:e20200005. doi: 10.1590/1678-9199-JVATITD-2020-0005. eCollection 2020. J Venom Anim Toxins Incl Trop Dis. 2020. PMID: 32742278 Free PMC article.
-
Negative inotropic mechanisms of β-cardiotoxin in cardiomyocytes by depression of myofilament ATPase activity without activation of the classical β-adrenergic pathway.Sci Rep. 2021 Oct 27;11(1):21154. doi: 10.1038/s41598-021-00282-x. Sci Rep. 2021. PMID: 34707114 Free PMC article.
References
-
- Ogawa T, Chijiwa T, Oda‐Ueda N, Ohno M (2005) Molecular diversity and accelerated evolution of C‐type lectin‐like proteins from snake venom. Toxicon 45:1–14. - PubMed
-
- Kini RM, Doley R (2010) Structure, function and evolution of three‐finger toxins: mini proteins with multiple targets. Toxicon 56:855–867. - PubMed
-
- Kang TS, Georgieva D, Genov N, Murakami MT, Sinha M, Kumar RP, Kaur P, Kumar S, Dey S, Sharma S, Vrielink A, Betzel C, Takeda S, Arni RK, Singh TP, Kini RM (2011) Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J 278:4544–4576. - PubMed
-
- Utkin YN (2013) Three‐finger toxins, a deadly weapon of elapid venom – milestones of discovery. Toxicon 62:50–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

