Secondary lymphoid tissue and costimulation-blockade resistant rejection: A nonhuman primate renal transplant study
- PMID: 30891931
- PMCID: PMC6658331
- DOI: 10.1111/ajt.15365
Secondary lymphoid tissue and costimulation-blockade resistant rejection: A nonhuman primate renal transplant study
Abstract
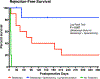
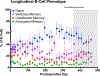
Naïve T cell activation requires antigen presentation combined with costimulation through CD28, both of which optimally occur in secondary lymphoid tissues such as lymph nodes and the spleen. Belatacept impairs CD28 costimulation by binding its ligands, CD80 and CD86, and in doing so, impairs de novo alloimmune responses. However, in most patients belatacept is ineffective in preventing allograft rejection when used as a monotherapy, and adjuvant therapy is required for control of costimulation-blockade resistant rejection (CoBRR). In rodent models, impaired access to secondary lymphoid tissues has been demonstrated to reduce alloimmune responses to vascularized allografts. Here we show that surgical maneuvers, lymphatic ligation, and splenectomy, designed to anatomically limit access to secondary lymphoid tissues, control CoBRR and facilitate belatacept monotherapy in a nonhuman primate model of kidney transplantation without adjuvant immunotherapy. We further demonstrate that animals sustained on belatacept monotherapy progressively develop an increasingly naïve T and B cell repertoire, an effect that is accelerated by splenectomy and lost at the time of belatacept withdrawal and rejection. These pilot data inform the role of secondary lymphoid tissues on the development of CoBRR and the use of costimulation molecule-focused therapies.
Keywords: animal models: nonhuman primate; antigen presentation/recognition; basic (laboratory) research/science; costimulation; immunobiology; immunosuppressant - fusion proteins and monoclonal antibodies: belatacept; immunosuppression/immune modulation; kidney transplantation/nephrology; lymphocyte biology: differentiation/maturation; translational research/science.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures



Similar articles
-
Belatacept-Resistant Rejection Is Associated With CD28+ Memory CD8 T Cells.Am J Transplant. 2017 Sep;17(9):2285-2299. doi: 10.1111/ajt.14349. Epub 2017 Jul 11. Am J Transplant. 2017. PMID: 28502128 Free PMC article.
-
Postdepletion Lymphocyte Reconstitution During Belatacept and Rapamycin Treatment in Kidney Transplant Recipients.Am J Transplant. 2016 Feb;16(2):550-64. doi: 10.1111/ajt.13469. Epub 2015 Oct 5. Am J Transplant. 2016. PMID: 26436448 Free PMC article.
-
IL-7 receptor heterogeneity as a mechanism for repertoire change during postdepletional homeostatic proliferation and its relation to costimulation blockade-resistant rejection.Am J Transplant. 2018 Mar;18(3):720-730. doi: 10.1111/ajt.14589. Epub 2017 Dec 12. Am J Transplant. 2018. PMID: 29136317 Free PMC article.
-
Selective Costimulation Blockade With Antagonist Anti-CD28 Therapeutics in Transplantation.Transplantation. 2019 Sep;103(9):1783-1789. doi: 10.1097/TP.0000000000002740. Transplantation. 2019. PMID: 30951014 Review.
-
Belatacept in kidney transplantation.Curr Opin Organ Transplant. 2012 Dec;17(6):640-7. doi: 10.1097/MOT.0b013e32835a4c0d. Curr Opin Organ Transplant. 2012. PMID: 23044530 Review.
Cited by
-
Preventive CTLA-4-Ig Treatment Reduces Hepatic Egg Load and Hepatic Fibrosis in Schistosoma mansoni-Infected Mice.Biomed Res Int. 2019 Dec 16;2019:1704238. doi: 10.1155/2019/1704238. eCollection 2019. Biomed Res Int. 2019. PMID: 31950032 Free PMC article.
-
The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates.Sci Transl Med. 2023 Aug 30;15(711):eadf6376. doi: 10.1126/scitranslmed.adf6376. Epub 2023 Aug 30. Sci Transl Med. 2023. PMID: 37647390 Free PMC article.
References
-
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine 1999;341(23):1725–1730. - PubMed
-
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10(3):535–546. - PubMed
-
- Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 2011;11(1):66–76. - PubMed
-
- Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant 2012;12(3):630–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

