Sodium butyrate and panobinostat induce apoptosis of chronic myeloid leukemia cells via multiple pathways
- PMID: 30891950
- PMCID: PMC6503025
- DOI: 10.1002/mgg3.613
Sodium butyrate and panobinostat induce apoptosis of chronic myeloid leukemia cells via multiple pathways
Abstract
Purpose: Histone deacetylase inhibitor (HDACI) is a novel therapeutic option for cancer. However, the effects of HDACIs on chronic myeloid leukemia (CML) and the underlying mechanisms are still unknown. The aim of this study was to investigate the effect and the mechanism-of-action of two HDACI members, sodium butyrate (NaBu) and panobinostat (LBH589) in K562 and the adriamycin-resistant cell line K562/ADR.
Methods: Cell viability was assessed using MTT assay. Cell apoptosis was detected with flow cytometry. Cell cycle analysis and western blot were performed to explore the possible molecules related to HDACIs effects.
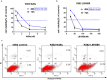
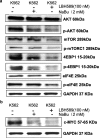
Results: The effect of NaBu was more powerful on K562/ADR than on K562 cells. LBH589 triggered apoptosis and inhibited the growth of K562 cells. Both HDACIs inhibited K562 and K562/ADR cells via activation of intrinsic/extrinsic apoptotic pathways and inhibition of AKT-mTOR pathway while NaBu also activated endoplasmic reticulum stress (ERS) mediated apoptotic pathway in K562/ADR cells. LBH589 reduced the expression of drug-resistant related proteins in K562 cells. However, neither NaBu nor LBH589 could significantly influence the expression of the drug-resistant related proteins in K562/ADR cells.
Conclusion: The combination of HDACI and other therapeutic strategies are likely required to overcome drug resistance in CML therapy.
Keywords: AKT-mTOR pathway; apoptosis; chronic myeloid leukemia (CML); drug resistance; histone deacetylase inhibitors (HDACIs).
© 2019 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors report no conflict of interest in this work.
Figures




Similar articles
-
HDAC1,2 Knock-Out and HDACi Induced Cell Apoptosis in Imatinib-Resistant K562 Cells.Int J Mol Sci. 2019 May 8;20(9):2271. doi: 10.3390/ijms20092271. Int J Mol Sci. 2019. PMID: 31071955 Free PMC article.
-
Abrogation of histone deacetylases (HDACs) decreases survival of chronic myeloid leukemia cells: New insight into attenuating effects of the PI3K/c-Myc axis on panobinostat cytotoxicity.Cell Biol Int. 2021 May;45(5):1111-1121. doi: 10.1002/cbin.11557. Epub 2021 Feb 4. Cell Biol Int. 2021. PMID: 33501756
-
Histone deacetylase inhibitors induce proteolysis of activated CDC42-associated kinase-1 in leukemic cells.J Cancer Res Clin Oncol. 2016 Nov;142(11):2263-73. doi: 10.1007/s00432-016-2229-x. Epub 2016 Aug 30. J Cancer Res Clin Oncol. 2016. PMID: 27576506 Free PMC article.
-
Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity.Adv Cancer Res. 2012;116:165-97. doi: 10.1016/B978-0-12-394387-3.00005-7. Adv Cancer Res. 2012. PMID: 23088871 Review.
-
Current treatment strategies targeting histone deacetylase inhibitors in acute lymphocytic leukemia: a systematic review.Front Oncol. 2024 Feb 21;14:1324859. doi: 10.3389/fonc.2024.1324859. eCollection 2024. Front Oncol. 2024. PMID: 38450195 Free PMC article. Review.
Cited by
-
HDAC inhibitor chidamide overcomes drug resistance in chronic myeloid leukemia with the T315i mutation through the Akt-autophagy pathway.Hum Cell. 2023 Jul;36(4):1564-1577. doi: 10.1007/s13577-023-00919-1. Epub 2023 May 24. Hum Cell. 2023. PMID: 37222919
-
Postbiotics are a candidate for new functional foods.Food Chem X. 2024 Jul 14;23:101650. doi: 10.1016/j.fochx.2024.101650. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39113733 Free PMC article. Review.
-
Sodium Butyrate Combined with Docetaxel for the Treatment of Lung Adenocarcinoma A549 Cells by Targeting Gli1.Onco Targets Ther. 2020 Sep 4;13:8861-8875. doi: 10.2147/OTT.S252323. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982280 Free PMC article.
-
Sodium Butyrate (NaB) and Sodium Propionate (NaP) Reduce Cyclin A2 Expression, Inducing Cell Cycle Arrest and Proliferation Inhibition of Different Breast Cancer Subtypes, Leading to Apoptosis.Biomedicines. 2024 Aug 6;12(8):1779. doi: 10.3390/biomedicines12081779. Biomedicines. 2024. PMID: 39200243 Free PMC article.
-
Histone deacetylase inhibitors for leukemia treatment: current status and future directions.Eur J Med Res. 2024 Oct 26;29(1):514. doi: 10.1186/s40001-024-02108-8. Eur J Med Res. 2024. PMID: 39456044 Free PMC article. Review.
References
-
- Bedi, A. , Barber, J. P. , Bedi, G. C. , el‐Deiry, W. S. , Sidransky, D. , Vala, M. S. , … Jones, R. J. (1995). BCR‐ABL‐mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: A mechanism of resistance to multiple anticancer agents. Blood, 86(3), 1148–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

