The role of the nAChR subunits α5, β2, and β4 on synaptic transmission in the mouse superior cervical ganglion
- PMID: 30891952
- PMCID: PMC6424856
- DOI: 10.14814/phy2.14023
The role of the nAChR subunits α5, β2, and β4 on synaptic transmission in the mouse superior cervical ganglion
Abstract
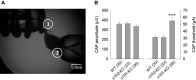
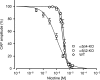
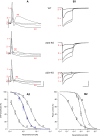
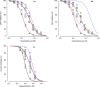
Our previous immunoprecipitation analysis of nicotinic acetylcholine receptors (nAChRs) in the mouse superior cervical ganglion (SCG) revealed that approximately 55%, 24%, and 21% of receptors are comprised of α3β4, α3β4α5, and α3β4β2 subunits, respectively. Moreover, mice lacking β4 subunits do not express α5-containing receptors but still express a small number of α3β2 receptors. Here, we investigated how synaptic transmission is affected in the SCG of α5β4-KO and α5β2-KO mice. Using an ex vivo SCG preparation, we stimulated the preganglionic cervical sympathetic trunk and measured compound action potentials (CAPs) in the postganglionic internal carotid nerve. We found that CAP amplitude was unaffected in α5β4-KO and α5β2-KO ganglia, whereas the stimulation threshold for eliciting CAPs was significantly higher in α5β4-KO ganglia. Moreover, intracellular recordings in SCG neurons revealed no difference in EPSP amplitude. We also found that the ganglionic blocking agent hexamethonium was the most potent in α5β4-KO ganglia (IC50 : 22.1 μmol/L), followed by α5β2-KO (IC50 : 126.7 μmol/L) and WT ganglia (IC50 : 389.2 μmol/L). Based on these data, we estimated an IC50 of 568.6 μmol/L for a receptor population consisting solely of α3β4α5 receptors; and we estimated that α3β4α5 receptors comprise 72% of nAChRs expressed in the mouse SCG. Similarly, by measuring the effects of hexamethonium on ACh-induced currents in cultured SCG neurons, we found that α3β4α5 receptors comprise 63% of nAChRs. Thus, in contrast to our results obtained using immunoprecipitation, these data indicate that the majority of receptors at the cell surface of SCG neurons consist of α3β4α5.
Keywords: EPSP; Compound action potential; Hexamethonium; knockout mice; nicotinic ACh receptor; superior cervical ganglion; synaptic transmission.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None declared.
Figures


References
-
- Bowers, C. W. , and Zigmond R. E.. 1979. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J. Comp. Neurol. 185:381–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

