Stereoselective Synthesis of Molecular Square and Granny Knots
- PMID: 30892025
- PMCID: PMC6492950
- DOI: 10.1021/jacs.9b01819
Stereoselective Synthesis of Molecular Square and Granny Knots
Abstract
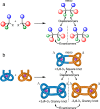
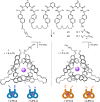
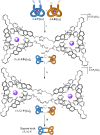
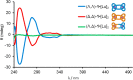
We report on the stereoselective synthesis of both molecular granny and square knots through the use of lanthanide-complexed overhand knots of specific handedness as three-crossing "entanglement synthons". The composite knots are assembled by combining two entanglement synthons (of the same chirality for a granny knot; of opposite handedness for a square knot) in three synthetic steps: first, a CuAAC reaction joins together one end of each overhand knot. Ring-closing olefin metathesis (RCM) then affords the closed-loop knot, locking the topology. This allows the lanthanide ions necessary for stabilizing the entangled conformation of the synthons to subsequently be removed. The composite knots were characterized by 1H and 13C NMR spectroscopy and mass spectrometry and the chirality of the knot stereoisomers compared by circular dichroism. The synthetic strategy of combining building blocks of defined stereochemistry (here overhand knots of Λ- or Δ-handed entanglement) is reminiscent of the chiron approach of using minimalist chiral synthons in the stereoselective synthesis of molecules with multiple asymmetric centers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Social Self-Sorting Synthesis of Molecular Knots.J Am Chem Soc. 2022 Sep 21;144(37):17232-17240. doi: 10.1021/jacs.2c07682. Epub 2022 Sep 6. J Am Chem Soc. 2022. PMID: 36067448 Free PMC article.
-
Tying a Molecular Overhand Knot of Single Handedness and Asymmetric Catalysis with the Corresponding Pseudo-D3-Symmetric Trefoil Knot.J Am Chem Soc. 2016 Oct 12;138(40):13159-13162. doi: 10.1021/jacs.6b08421. Epub 2016 Sep 29. J Am Chem Soc. 2016. PMID: 27667319 Free PMC article.
-
Folding a Molecular Strand into a Trefoil Knot of Single Handedness with Co(II)/Co(III) Chaperones.J Am Chem Soc. 2024 Aug 7;146(31):21762-21768. doi: 10.1021/jacs.4c05953. Epub 2024 Jul 26. J Am Chem Soc. 2024. PMID: 39060953 Free PMC article.
-
Significance of the internal locking mechanism for loop security enhancement in the arthroscopic knot.Arthroscopy. 2001 Oct;17(8):850-5. doi: 10.1016/s0749-8063(01)90009-x. Arthroscopy. 2001. PMID: 11600983 Review.
-
Molecular Knots.Angew Chem Int Ed Engl. 2017 Sep 4;56(37):11166-11194. doi: 10.1002/anie.201702531. Epub 2017 Aug 16. Angew Chem Int Ed Engl. 2017. PMID: 28477423 Free PMC article. Review.
Cited by
-
A Co-conformationally "Topologically" Chiral Catenane.J Am Chem Soc. 2022 Jul 13;144(27):11927-11932. doi: 10.1021/jacs.2c02029. Epub 2022 Jun 28. J Am Chem Soc. 2022. PMID: 35763555 Free PMC article.
-
Knotting a molecular strand can invert macroscopic effects of chirality.Nat Chem. 2020 Oct;12(10):939-944. doi: 10.1038/s41557-020-0517-1. Epub 2020 Aug 3. Nat Chem. 2020. PMID: 32747756
-
Channels with Helical Modulation Display Stereospecific Sensitivity for Chiral Superstructures.Polymers (Basel). 2021 Oct 28;13(21):3726. doi: 10.3390/polym13213726. Polymers (Basel). 2021. PMID: 34771282 Free PMC article.
-
Effects of turn-structure on folding and entanglement in artificial molecular overhand knots.Chem Sci. 2020 Dec 8;12(5):1826-1833. doi: 10.1039/d0sc05897a. Chem Sci. 2020. PMID: 34163946 Free PMC article.
-
Distinctive features and challenges in catenane chemistry.Chem Sci. 2022 Feb 7;13(12):3315-3334. doi: 10.1039/d1sc05391d. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432874 Free PMC article. Review.
References
-
- Fielden S. D. P.; Leigh D. A.; Woltering S. L. Molecular knots. Angew. Chem., Int. Ed. 2017, 56, 11166–11194. 10.1002/anie.201702531. - DOI - PMC - PubMed
-
For other reviews on molecular knots, see:
- Fenlon E. E. Open problems in chemical topology. Eur. J. Org. Chem. 2008, 2008, 5023–5035. 10.1002/ejoc.200800578. - DOI
- Beves J. E.; Blight B. A.; Campbell C. J.; Leigh D. A.; McBurney R. T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem., Int. Ed. 2011, 50, 9260–9327. 10.1002/anie.201007963. - DOI - PubMed
- Forgan R. S.; Sauvage J.-P.; Stoddart J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 2011, 111, 5434–5464. 10.1021/cr200034u. - DOI - PubMed
- Amabilino D. B.; Sauvage J.-P. The beauty of knots at the molecular level. Top. Curr. Chem. 2011, 323, 107–126. 10.1007/128_2011_292. - DOI - PubMed
- Ayme J.-F.; Beves J. E.; Campbell C. J.; Leigh D. A. Template synthesis of molecular knots. Chem. Soc. Rev. 2013, 42, 1700–1712. 10.1039/C2CS35229J. - DOI - PubMed
- Horner K. E.; Miller M. A.; Steed J. W.; Sutcliffe P. M. Knot theory in modern chemistry. Chem. Soc. Rev. 2016, 45, 6432–6448. 10.1039/C6CS00448B. - DOI - PubMed
- Sauvage J.-P. From chemical topology to molecular machines (Nobel lecture). Angew. Chem., Int. Ed. 2017, 56, 11080–11093. 10.1002/anie.201702992. - DOI - PubMed
- Pairault N.; Niemeyer J. Chiral mechanically interlocked molecules – Applications of rotaxanes, catenanes and molecular knots in stereoselective chemosensing and catalysis. Synlett 2018, 29, 689–698. 10.1055/s-0036-1591934. - DOI
-
- Dietrich-Buchecker C. O.; Sauvage J.-P. A synthetic molecular trefoil knot. Angew. Chem., Int. Ed. Engl. 1989, 28, 189–192. 10.1002/anie.198901891. - DOI
- Ashton P. R.; Matthews O. A.; Menzer S.; Raymo F. M.; Spencer N.; Stoddart J. F.; Williams D. J. A template-directed synthesis of a molecular trefoil knot. Liebigs Ann. Chem. 1997, 1997, 2485–2494. 10.1002/jlac.199719971210. - DOI
- Dietrich-Buchecker C. O.; Hwang N. G.; Sauvage J.-P. A trefoil knot coordinated to two lithium ions: synthesis and structure. New J. Chem. 1999, 23, 911–914. 10.1039/a904476k. - DOI
- Safarowsky O.; Nieger M.; Fröhlich R.; Vögtle F. A molecular knot with twelve amide groups—one-step synthesis, crystal structure, chirality. Angew. Chem., Int. Ed. 2000, 39, 1616–1618. 10.1002/(SICI)1521-3773(20000502)39:9<1616::AID-ANIE1616>3.0.CO;2-Y. - DOI - PubMed
- Feigel M.; Ladberg R.; Engels S.; Herbst-Irmer R.; Fröhlich R. A trefoil knot made of amino acids and steroids. Angew. Chem., Int. Ed. 2006, 45, 5698–5702. 10.1002/anie.200601111. - DOI - PubMed
- Guo J.; Mayers P. C.; Breault G. A.; Hunter C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2010, 2, 218–220. 10.1038/nchem.544. - DOI - PubMed
- Barran P. E.; Cole H. L.; Goldup S. M.; Leigh D. A.; McGonigal P. R.; Symes M. D.; Wu J. Y.; Zengerle M. Active metal template synthesis of a molecular trefoil knot. Angew. Chem., Int. Ed. 2011, 50, 12280–12284. 10.1002/anie.201105012. - DOI - PubMed
- Ponnuswamy N.; Cougnon F. B. L.; Clough J. M.; Pantoş G. D.; Sanders J. K. M. Discovery of an organic trefoil knot. Science 2012, 338, 783–785. 10.1126/science.1227032. - DOI - PubMed
- Prakasam T.; Lusi M.; Elhabiri M.; Platas-Iglesias C.; Olsen J.-C.; Asfari Z.; Cianférani-Sanglier S.; Debaene F.; Charbonnière L. J.; Trabolsi A. Simultaneous self assembly of a [2]catenane, a trefoil knot, and a solomon link from a simple pair of ligands. Angew. Chem. 2013, 125, 10140–10144. 10.1002/ange.201302425. - DOI - PubMed
- Ayme J.-F.; Gil-Ramírez G.; Leigh D. A.; Lemonnier J.-F.; Markevicius A.; Muryn C. A.; Zhang G. Lanthanide template synthesis of a molecular trefoil knot. J. Am. Chem. Soc. 2014, 136, 13142–13145. 10.1021/ja506886p. - DOI - PubMed
- Prakasam T.; Bilbeisi R. A.; Lusi M.; Olsen J.-C.; Platas-Iglesias C.; Trabolsi A. Postsynthetic modification of cadmium-based knots and links. Chem. Commun. 2016, 52, 7398–7401. 10.1039/C6CC02423H. - DOI - PubMed
- Zhang L.; August D. P.; Zhong J.; Whitehead G. F. S.; Vitorica-Yrezabal I. J.; Leigh D. A. Molecular trefoil knot from a trimeric circular helicate. J. Am. Chem. Soc. 2018, 140, 4982–4985. 10.1021/jacs.8b00738. - DOI - PubMed
- Cougnon F. B. L.; Caprice K.; Pupier M.; Bauzá A.; Frontera A. A strategy to synthesize molecular knots and links using the hydrophobic effect. J. Am. Chem. Soc. 2018, 140, 12442–12450. 10.1021/jacs.8b05220. - DOI - PubMed
-
- Adams C. C.The Knot Book: An Elementary Introduction to the Mathematical Theory of Knots; American Mathematical Society: Providence, RI, 2004.
-
- Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15–20. 10.1038/nchem.1193. - DOI - PubMed
- Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. Pentameric circular iron(II) double helicates and a molecular pentafoil knot. J. Am. Chem. Soc. 2012, 134, 9488–9497. 10.1021/ja303355v. - DOI - PubMed
- Ponnuswamy N.; Cougnon F. B. L.; Pantoş G. D.; Sanders J. K. M. Homochiral and meso figure eight knots and a Solomon link. J. Am. Chem. Soc. 2014, 136, 8243–8251. 10.1021/ja4125884. - DOI - PubMed
- Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Allosteric initiation and regulation of catalysis with a molecular knot. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. - DOI - PubMed
- Danon J. J.; Krüger A.; Leigh D. A.; Lemonnier J.-F.; Stephens A. J.; Vitorica-Yrezabal I. J.; Woltering S. L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. 10.1126/science.aal1619. - DOI - PubMed
- Cougnon F. B. L. Tight embrace in a molecular knot with eight crossings. Angew. Chem., Int. Ed. 2017, 56, 4918–4919. 10.1002/anie.201701308. - DOI - PubMed
- Kim D. H.; Singh N.; Oh J.; Kim E.-H.; Jung J.; Kim H.; Chi K.-W. Coordination-driven self-assembly of a molecular knot comprising sixteen crossings. Angew. Chem., Int. Ed. 2018, 57, 5669–5673. 10.1002/anie.201800638. - DOI - PubMed
- Leigh D. A.; Lemonnier J.-F.; Woltering S. L. Comment on “Coordination-driven self-assembly of a molecular knot comprising sixteen crossings. Angew. Chem., Int. Ed. 2018, 57, 12212–12214. 10.1002/anie.201804904. - DOI - PubMed
- Zhang L.; Lemonnier J.-F.; Acocella A.; Calvaresi M.; Zerbetto F.; Leigh D. A. Effects of knot tightness at the molecular level. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2452.10.1073/pnas.1815570116. - DOI - PMC - PubMed
-
- Carina R. F.; Dietrich-Buchecker C. O.; Sauvage J.-P. Molecular composite knots. J. Am. Chem. Soc. 1996, 118, 9110–9116. 10.1021/ja961459p. - DOI
- Danon J. J.; Leigh D. A.; Pisano S.; Valero A.; Vitorica-Yrezabal I. J. A Six-Crossing Doubly Interlocked [2]Catenane with Twisted Rings, and a Molecular Granny Knot. Angew. Chem., Int. Ed. 2018, 57, 13833–13837. 10.1002/anie.201807135. - DOI - PMC - PubMed
- Zhang L.; Stephens A. J.; Nussbaumer A. L.; Lemonnier J.-F.; Jurček P.; Vitorica-Yrezabal I. J.; Leigh D. A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. 10.1038/s41557-018-0124-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

