Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice
- PMID: 30892078
- PMCID: PMC6620668
- DOI: 10.1152/ajplung.00302.2018
Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice
Abstract
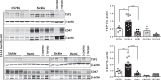
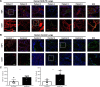
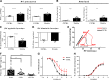
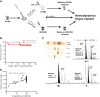
Pulmonary hypertension (PH) is a leading cause of death in sickle cell disease (SCD) patients. Hemolysis and oxidative stress contribute to SCD-associated PH. We have reported that the protein thrombospondin-1 (TSP1) is elevated in the plasma of patients with SCD and, by interacting with its receptor CD47, limits vasodilation of distal pulmonary arteries ex vivo. We hypothesized that the TSP1-CD47 interaction may promote PH in SCD. We found that TSP1 and CD47 are upregulated in the lungs of Berkeley (BERK) sickling (Sickle) mice and patients with SCD-associated PH. We then generated chimeric animals by transplanting BERK bone marrow into C57BL/6J (n = 24) and CD47 knockout (CD47KO, n = 27) mice. Right ventricular (RV) pressure was lower in fully engrafted Sickle-to-CD47KO than Sickle-to-C57BL/6J chimeras, as shown by the reduced maximum RV pressure (P = 0.013) and mean pulmonary artery pressure (P = 0.020). The afterload of the sickle-to-CD47KO chimeras was also lower, as shown by the diminished pulmonary vascular resistance (P = 0.024) and RV effective arterial elastance (P = 0.052). On myography, aortic segments from Sickle-to-CD47KO chimeras showed improved relaxation to acetylcholine. We hypothesized that, in SCD, TSP1-CD47 signaling promotes PH, in part, by increasing reactive oxygen species (ROS) generation. In human pulmonary artery endothelial cells, treatment with TSP1 stimulated ROS generation, which was abrogated by CD47 blockade. Explanted lungs of CD47KO chimeras had less vascular congestion and a smaller oxidative footprint. Our results show that genetic absence of CD47 ameliorates SCD-associated PH, which may be due to decreased ROS levels. Modulation of TSP1-CD47 may provide a new molecular approach to the treatment of SCD-associated PH.
Keywords: oxidant stress; pulmonary hypertension; sickle cell disease; thrombospondin-1; transgenic mouse models.
Conflict of interest statement
J. S. Isenberg serves as Chair of the Scientific Advisory Board of Radiation Control Technologies, Inc. (RCTI, Garden City, NJ) and has equity interest in RCTI and Tioma Therapeutics (St. Louis, MO) that have licensed CD47 technology for development. M. T. Gladwin is a co-inventor on pending patent applications and planned patents directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning, which have recently been licensed by Globin Solutions, Inc. M. T. Gladwin is a shareholder, advisor, and director in Globin Solutions, Inc. Additionally, and unrelated to CO poisoning, M. T. Gladwin is a co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which have been licensed by United Therapeutics and Hope Pharmaceuticals, and is a co-investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguate as a treatment for patients with SCD. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



References
-
- Al Ghouleh I, Meijles DN, Mutchler S, Zhang Q, Sahoo S, Gorelova A, Henrich Amaral J, Rodríguez AI, Mamonova T, Song GJ, Bisello A, Friedman PA, Cifuentes-Pagano ME, Pagano PJ. Binding of EBP50 to Nox organizing subunit p47phox is pivotal to cellular reactive species generation and altered vascular phenotype. Proc Natl Acad Sci USA 113: E5308–E5317, 2016. doi:10.1073/pnas.1514161113. - DOI - PMC - PubMed
-
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001. doi:10.1073/pnas.221292098. - DOI - PMC - PubMed
-
- Bakeer N, James J, Roy S, Wansapura J, Shanmukhappa SK, Lorenz JN, Osinska H, Backer K, Huby AC, Shrestha A, Niss O, Fleck R, Quinn CT, Taylor MD, Purevjav E, Aronow BJ, Towbin JA, Malik P. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci USA 113: E5182–E5191, 2016. doi:10.1073/pnas.1600311113. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

