Evaluation of Low-Dose, Low-Frequency Oral Psoralen-UV-A Treatment With or Without Maintenance on Early-Stage Mycosis Fungoides: A Randomized Clinical Trial
- PMID: 30892603
- PMCID: PMC6506892
- DOI: 10.1001/jamadermatol.2018.5905
Evaluation of Low-Dose, Low-Frequency Oral Psoralen-UV-A Treatment With or Without Maintenance on Early-Stage Mycosis Fungoides: A Randomized Clinical Trial
Erratum in
-
Wording Errors in Abstract, Numeric Errors in Results, and Labeling Errors in Figure 2.JAMA Dermatol. 2019 May 1;155(5):638. doi: 10.1001/jamadermatol.2019.0773. JAMA Dermatol. 2019. PMID: 31066883 Free PMC article. No abstract available.
-
Error in Title of Letter to the Editor.JAMA Dermatol. 2019 Oct 1;155(10):1201. doi: 10.1001/jamadermatol.2019.2181. JAMA Dermatol. 2019. PMID: 31596444 Free PMC article. No abstract available.
Abstract
Importance: Psoralen-UV-A (PUVA) photochemotherapy is standard first-line treatment for skin-limited, early-stage mycosis fungoides capable of producing high initial complete response (CR) rates. However, much remains unknown about PUVA's therapeutic mechanisms, optimal duration and frequency of treatment, dose escalation, or use as maintenance therapy.
Objectives: To evaluate low-dose, low-frequency PUVA, and whether maintenance treatment extends disease-free remission in patients with mycosis fungoides.
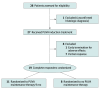
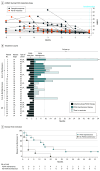
Design, setting, and participants: This prospective randomized clinical trial with defined PUVA dosing regimen was carried out in 5 centers (Graz, Vienna, Hietzing, Innsbruck, and Salzburg) across Austria. Patients with stage IA to IIA mycosis fungoides (n = 27) were enrolled in the study beginning March 13, 2013, with the last patient enrolled March 21, 2016. These patients were treated with oral 8-methoxypsoralen followed by UV-A exposure 2 times per week for 12 to 24 weeks until CR. Patients with CR were randomized to PUVA maintenance for 9 months (14 total exposures) or no maintenance. The study was conducted from April 27, 2012, to July 27, 2018. Data analysis of the primary end point was of the intention-to-treat population, and the secondary end point analysis was of the evaluable population.
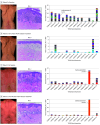
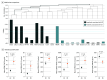
Main outcomes and measures: Efficacy of the PUVA regimen was determined by the rate of CR as defined by a modified severity-weighted assessment tool (mSWAT) score reduction to 0. Levels of proinflammatory molecules in serum and histologic features and percentage of clonal T cells in skin were assessed to search for biomarkers of clinical response.
Results: In 27 patients with mycosis fungoides, 19 (70%) were male with mean (range) age 61 (30-80) years. At baseline, patients with CR had a mean (range) mSWAT score of 18.6 (1-66) compared with 16.8 (3-46) in patients with partial response. The 12- to 24-week PUVA induction regimen reduced the mSWAT score in all patients and led to CR in 19 (70%) of 27 patients and a low mean cumulative UV-A dose of 78.5 J/cm2. The subsequent standardized 9-month PUVA maintenance phase prolonged median (range) disease-free remission from 4 (1-20) months to 15 (1-54) months (P = .02). High density of histologic infiltrate and high percentage of clonal TCR sequences in skin biopsy specimens at baseline were inversely associated with therapeutic response. No severe adverse effects were seen during the PUVA induction or maintenance phase.
Conclusions and relevance: This proof-of-concept study identifies potential biomarkers for therapeutic response to PUVA in mycosis fungoides; it also demonstrates that low-dose, low-frequency PUVA appears to be highly effective, and maintenance treatment may extend disease-free remission.
Trial registration: ClinicalTrials.gov identifier: NCT01686594.
Conflict of interest statement
Figures
Comment in
-
Psoralen Plus UV-A Therapy in the 21st Century: Use It or Lose It.JAMA Dermatol. 2019 May 1;155(5):529-531. doi: 10.1001/jamadermatol.2018.5844. JAMA Dermatol. 2019. PMID: 30892578 No abstract available.
-
Explanation of Additional Errors in Report of Trial of Low-Dose, Low-Frequency Oral Psoralen-UV-A Treatment.JAMA Dermatol. 2019 Oct 1;155(10):1200-1201. doi: 10.1001/jamadermatol.2019.2698. JAMA Dermatol. 2019. PMID: 31596447 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

