Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis
- PMID: 30892837
- PMCID: PMC6640101
- DOI: 10.1002/alr.22334
Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis
Abstract
Background: Solitary chemosensory cells (SCCs) are rare epithelial cells enriched in nasal polyps and are the primary source of interleukin-25 (IL-25), an innate cytokine eliciting T-helper 2 (Th2) immune response. Although it is proposed that SCCs are stimulated by antigens released by upper airway pathogens, the exogenous triggers of human SCCs remain elusive. We studied patients with noninvasive fungal rhinosinusitis to determine whether extracts of Aspergillus fumigatus and Alternaria alternata stimulate SCC proliferation as an early event in type 2 inflammation.
Methods: Multicolor flow cytometry, immunofluorescence, and enzyme-linked immunoassay were used to interrogate mucosa from patients with mycetomas and allergic fungal rhinosinusitis (AFRS) for SCCs and IL-25. Primary sinonasal epithelial cells from AFRS patients and noninflamed inferior turbinates were stimulated with fungal extracts for 72 hours, and SCC population frequency as well as mitotic activity were quantified using flow cytometry.
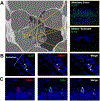
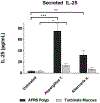
Results: SCCs producing IL-25 are enriched in inflamed mucosa compared with intrapatient noninflamed control tissue (38.6% vs 6.5%, p = 0.029). In cultured sinonasal epithelial cells from AFRS nasal polyps, Aspergillus fumigatus and Alternaria alternata stimulated higher SCC frequency compared with controls (27.4% vs 10.6%, p = 0.002; 18.1% vs 10.6%, p = 0.046), which led to increased IL-25 secretion in culture media (75.5 vs 3.3 pg/mL, p < 0.001; 32.3 vs 3.3 pg/mL, p = 0.007). Ki-67 expression was higher in SCCs grown in fungal stimulation conditions compared with controls.
Conclusion: Although fungal antigens are known to potentiate immune response through innate cytokines, including IL-25, the early expansion of SCCs in the presence of fungus has not been described. This early event in the pathogenesis of noninvasive fungal rhinosinusitis may represent a target for intervention.
Keywords: IL-25; allergic fungal rhinosinusitis; fungal antigens; mycetoma; solitary chemosensory cells; type 2 inflammation.
© 2019 ARS-AAOA, LLC.
Figures

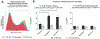
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC013588/DC/NIDCD NIH HHS/United States
- TL1TR001880 (to NNP)/TR/NCATS NIH HHS/United States
- R01DC013588 (to NAC)/Efficacy and Mechanism Evaluation Programme/International
- I01 CX001617/CX/CSRD VA/United States
- R01 AI095289/AI/NIAID NIH HHS/United States
- UO1AI125940 (to DRH)/Efficacy and Mechanism Evaluation Programme/International
- R01 GM083204/GM/NIGMS NIH HHS/United States
- R01AI095289 (to DRH)/Efficacy and Mechanism Evaluation Programme/International
- GM083204-08A1 (to DRH)/Efficacy and Mechanism Evaluation Programme/International
- U01 AI125940/AI/NIAID NIH HHS/United States
- TL1 TR001880/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

