Ruthenium-Catalyzed Redox Isomerizations inside Living Cells
- PMID: 30892889
- PMCID: PMC6497367
- DOI: 10.1021/jacs.9b00837
Ruthenium-Catalyzed Redox Isomerizations inside Living Cells
Abstract
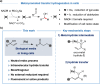
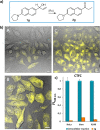
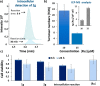
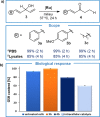
Tailored ruthenium(IV) complexes can catalyze the isomerization of allylic alcohols into saturated carbonyl derivatives under physiologically relevant conditions, and even inside living mammalian cells. The reaction, which involves ruthenium-hydride intermediates, is bioorthogonal and biocompatible, and can be used for the "in cellulo" generation of fluorescent and bioactive probes. Overall, our research reveals a novel metal-based tool for cellular intervention, and comes to further demonstrate the compatibility of organometallic mechanisms with the complex environment of cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Berg J. M.; Tymoczko J. L.; Stryer L.. Biochemistry; W. H. Freeman: New York, 2002.
- Garcia-Viloca M.; Gao J.; Karplus M.; Truhlar D. G. How enzymes work: analysis by modern rate theory and computer simulations. Science 2004, 303, 186–195. 10.1126/science.1088172. - DOI - PubMed
- Küchler A.; Yoshimoto M.; Luginbühl S.; Mavelli F.; Walde P. Enzymatic reactions in confined enviornments. Nat. Nanotechnol. 2016, 11, 409–420. 10.1038/nnano.2016.54. - DOI - PubMed
- Agarwal P. K. Biophysical perspective on enzyme catalysis. Biochemistry 2019, 58, 438–449. 10.1021/acs.biochem.8b01004. - DOI - PMC - PubMed
-
- Hegedus L. S.Transition metals in the synthesis of complex organic molecules; Wiley-VCH: Weinheim, 1999.
- Beller M.; Bolm C.. Transition metals for organic synthesis: building blocks and fine chemicals; Wiley-VCH: Weinheim, 2008.
- Hartwig J. F.Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Mill Valley, CA, 2010.
-
- Sasmal P. K.; Streu C. N.; Meggers E. Metal complex catalysis in living biological systems. Chem. Commun. 2013, 49, 1581–1587. 10.1039/C2CC37832A. - DOI - PubMed
- Yang M.; Li J.; Chen R. P. Transition metal-mediated bioorthogonal protein chemistry in living cells. Chem. Soc. Rev. 2014, 43, 6511–6526. 10.1039/C4CS00117F. - DOI - PubMed
- Vinogradova E. V. Organometallic chemical biology: an organometallic approach to bioconjugation. Pure Appl. Chem. 2017, 89, 1619–1641. 10.1515/pac-2017-0207. - DOI
- Rebelein J. G.; Ward T. R. In vivo catalyzed new-to-nature reactions. Curr. Opin. Biotechnol. 2018, 53, 106–114. 10.1016/j.copbio.2017.12.008. - DOI - PubMed
- Martínez-Calvo M.; Mascareñas J. L. Organometallic catalysis in biological media and living settings. Coord. Chem. Rev. 2018, 359, 57–79. 10.1016/j.ccr.2018.01.011. - DOI
- Ngo A. H.; Bose S.; Do L. H. Intracellular chemistry: integrating molecular inorganic catalysts with living systems. Chem. - Eur. J. 2018, 24, 10584–10594. 10.1002/chem.201800504. - DOI - PubMed
- Bai Y.; Chen J.; Zimmerman S. C. Designed transition metal catalysts for intracellular organic synthesis. Chem. Soc. Rev. 2018, 47, 1811–1821. 10.1039/C7CS00447H. - DOI - PubMed
- Soldevilla-Barreda J. J.; Metzler-Nolte N. Intracelullar catalysis with selected metal complexes and metallic nanoparticles: advances toward the development of catalytic metallodrugs. Chem. Rev. 2019, 119, 829–869. 10.1021/acs.chemrev.8b00493. - DOI - PubMed
-
- Streu C.; Meggers E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem., Int. Ed. 2006, 45, 5645–5648. 10.1002/anie.200601752. - DOI - PubMed
- Yusop R. M.; Unciti-Broceta A.; Johansson E. M. V.; Sánchez-Martín R. M.; Bradley M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239–243. 10.1038/nchem.981. - DOI - PubMed
- Völker T.; Dempwolff F.; Graumann P. L.; Meggers E. Progress towards bioorthogonal catalysis with organometallic compounds. Angew. Chem., Int. Ed. 2014, 53, 10536–10540. 10.1002/anie.201404547. - DOI - PubMed
- Sánchez M. I.; Penas C.; Vázquez M. E.; Mascareñas J. L. Metal-catalyzed uncaging of DNA-binding agents in living cells. Chem. Sci. 2014, 5, 1901–1907. 10.1039/C3SC53317D. - DOI - PMC - PubMed
- Tonga G. Y.; Jeong Y.; Duncan B.; Mizuhara T.; Mout R.; Das R.; Kim S. T.; Yeh Y.-C.; Yan B.; Hou S.; Rotello V. M. Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat. Chem. 2015, 7, 597–603. 10.1038/nchem.2284. - DOI - PMC - PubMed
- Tomás-Gamasa M.; Martínez-Calvo M.; Couceiro J. M.; Mascareñas J. L. Transition metal catalysis in the mitochondria of living cells. Nat. Commun. 2016, 7, 12538–12547. 10.1038/ncomms12538. - DOI - PMC - PubMed
- Wang J.; Zheng S.; Liu Y.; Zhang Z.; Lin Z.; Li J.; Zhang G.; Wang X.; Li J.; Chen P. R. Palladium-triggered chemical rescue of intracellular proteins via genetically encoded allene-caged tyrosine. J. Am. Chem. Soc. 2016, 138, 15118–15121. 10.1021/jacs.6b08933. - DOI - PubMed
- Miller M. A.; Askevold B.; Mikula H.; Kohler R. H.; Pirovich D.; Weissleder R. Nano-palladium is a cellular catalyst for in vivo chemistry. Nat. Commun. 2017, 8, 15906–15918. 10.1038/ncomms15906. - DOI - PMC - PubMed
- Stenton B. J.; Oliveira B. L.; Matos M. J.; Sinatra L.; Bernardes G. J. L. A thioether-directed palladium-cleavable linker for targeted bioorthogonal drug decaging. Chem. Sci. 2018, 9, 4185–4189. 10.1039/C8SC00256H. - DOI - PMC - PubMed
- Martínez-Calvo M.; Couceiro J. R.; Destito P.; Rodríguez J.; Mosquera J.; Mascareñas J. L. Intracellular deprotection reactions mediated by palladium complexes equipped with designed phosphine ligands. ACS Catal. 2018, 8, 6055–6061. 10.1021/acscatal.8b01606. - DOI - PMC - PubMed
-
- Clavadetscher J.; Hoffmann S.; Lilienkampf A.; Mackay L.; Yusop R. M.; Rider S. A.; Mullins J. J.; Bradley M. Copper catalysis in living systems and in situ drug synthesis. Angew. Chem., Int. Ed. 2016, 55, 15662–15666. 10.1002/anie.201609837. - DOI - PubMed
- Li S.; Wang L.; Yu F.; Zhu Z.; Shobaki D.; Chen H.; Wang M.; Wang J.; Qin G.; Erasquin U. J.; Ren L.; Wang Y.; Cai C. Copper-catalyzed click reaction on/in live cells. Chem. Sci. 2017, 8, 2107–2114. 10.1039/C6SC02297A. - DOI - PMC - PubMed
- Destito P.; Couceiro J. R.; Faustino H.; López F.; Mascareñas J. L. Ruthenium-catalyzed azide-thioalkyne cycloadditions in aqueous media: a mild, orthogonal, and biocompatible chemical ligation. Angew. Chem., Int. Ed. 2017, 56, 10766–10770. 10.1002/anie.201705006. - DOI - PMC - PubMed
- Miguel-Ávila J.; Tomás-Gamasa M.; Olmos A.; Pérez P. J.; Mascareñas J. L. Discrete Cu(I) complexes azide-alkyne annulations of small molecules inside mammalian cells. Chem. Sci. 2018, 9, 1947–1952. 10.1039/C7SC04643J. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

