Angiotensin type 1a receptors in the median preoptic nucleus support intermittent hypoxia-induced hypertension
- PMID: 30892911
- PMCID: PMC6589598
- DOI: 10.1152/ajpregu.00393.2018
Angiotensin type 1a receptors in the median preoptic nucleus support intermittent hypoxia-induced hypertension
Abstract
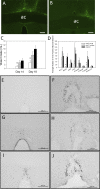
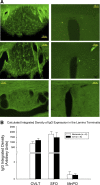
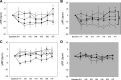
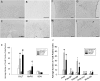
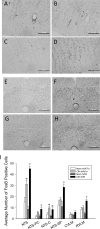
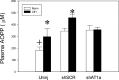
Chronic intermittent hypoxia (CIH) is a model of the hypoxemia from sleep apnea that causes a sustained increase in blood pressure. Inhibition of the central renin-angiotensin system or FosB in the median preoptic nucleus (MnPO) prevents the sustained hypertensive response to CIH. We tested the hypothesis that angiotensin type 1a (AT1a) receptors in the MnPO, which are upregulated by CIH, contribute to this hypertension. In preliminary experiments, retrograde tract tracing studies showed AT1a receptor expression in MnPO neurons projecting to the paraventricular nucleus. Adult male rats were exposed to 7 days of intermittent hypoxia (cycling between 21% and 10% O2 every 6 min, 8 h/day during light phase). Seven days of CIH was associated with a FosB-dependent increase in AT1a receptor mRNA without changes in the permeability of the blood-brain barrier in the MnPO. Separate groups of rats were injected in the MnPO with an adeno-associated virus containing short hairpin (sh)RNA against AT1a receptors to test their role in intermittent hypoxia hypertension. Injections of shRNA against AT1a in MnPO blocked the increase in mRNA associated with CIH, prevented the sustained component of the hypertension during normoxia, and reduced circulating advanced oxidation protein products, an indicator of oxidative stress. Rats injected with shRNA against AT1a and exposed to CIH had less FosB staining in MnPO and the rostral ventrolateral medulla after intermittent hypoxia than rats injected with the control vector that were exposed to CIH. Our results indicate AT1a receptors in the MnPO contribute to the sustained blood pressure increase to intermittent hypoxia.
Keywords: angiotensin; hypertension; sleep apnea.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Bathina CS, Rajulapati A, Franzke M, Yamamoto K, Cunningham JT, Mifflin S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1031–R1039, 2013. doi:10.1152/ajpregu.00260.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

