Identification of transmembrane protein 237 as a novel interactor with the intestinal riboflavin transporter-3 (RFVT-3): role in functionality and cell biology
- PMID: 30892938
- PMCID: PMC6620576
- DOI: 10.1152/ajpcell.00029.2019
Identification of transmembrane protein 237 as a novel interactor with the intestinal riboflavin transporter-3 (RFVT-3): role in functionality and cell biology
Abstract
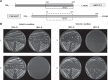
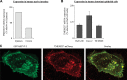
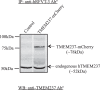
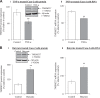
The apically localized riboflavin (RF) transporter-3 (RFVT-3) is involved in intestinal absorption of vitamin B2. Previous studies have characterized different physiological/biological aspects of the RFVT-3, but there is a lack of knowledge regarding possible existence of interacting partner(s) and consequence of interaction(s) on its function/cell biology. To address the latter, we performed yeast two-hybrid (Y2H) screening of a human colonic cDNA library and have identified transmembrane protein 237 (TMEM237) as a putative interactor with the human (h)RFVT-3; the interaction was further confirmed via "1-by-1" Y2H assay that involved appropriate positive and negative controls. TMEM237 was found to be highly expressed in human native intestine and in human intestinal epithelial cell lines; further, confocal images showed colocalization of the protein with hRFVT-3. The interaction between TMEM237 with hRFVT-3 in human intestinal epithelial HuTu-80 cells was established by coimmunoprecipitation. Expressing TMEM237 in HuTu-80 cells led to a significant induction in RF uptake, while its knockdown (with the use of gene-specific siRNA) led to a significant reduction in uptake. Transfecting TMEM237 into HuTu-80 cells also led to a marked enhancement in hRFVT-3 protein stability (reflected by an increase in the protein half-life). Interestingly, the level of expression of TMEM237 was found to be markedly reduced following treatment with TNF-α (a proinflammatory cytokine that inhibits intestinal RF uptake), while its expression was significantly upregulated following treatment with butyrate (an inducer of intestinal RF uptake). These findings identify TMEM237 as an interactor with the intestinal hRFVT-3 and show that the interaction has physiological/biological significance.
Keywords: RFVT-3; TMEM237; interacting partner; intestine; riboflavin transporter.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Anandam KY, Alwan OA, Subramanian VS, Srinivasan P, Kapadia R, Said HM. Effect of the proinflammatory cytokine TNF-α on intestinal riboflavin uptake: inhibition mediated via transcriptional mechanism(s). Am J Physiol Cell Physiol 315: C653–C663, 2018. doi: 10.1152/ajpcell.00295.2018. - DOI - PMC - PubMed
-
- Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980. - PubMed
-
- Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis 34: 159–164, 2011. doi: 10.1007/s10545-010-9242-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

