Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study
- PMID: 30892987
- PMCID: PMC7370699
- DOI: 10.1200/JCO.18.02459
Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study
Abstract
Purpose: To determine the safety and preliminary efficacy of selective combination targeted therapy for BRAF V600E-mutant metastatic colorectal cancer (mCRC) in the safety lead-in phase of the open-label, randomized, three-arm, phase III BEACON Colorectal Cancer trial ( ClinicalTrials.gov identifier: NCT02928224; European Union Clinical Trials Register identifier: EudraCT2015-005805-35).
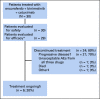
Patients and methods: Before initiation of the randomized portion of the BEACON Colorectal Cancer trial, 30 patients with BRAF V600E-mutant mCRC who had experienced treatment failure with one or two prior regimens were to be recruited to a safety lead-in of encorafenib 300 mg daily, binimetinib 45 mg twice daily, plus standard weekly cetuximab. The primary end point was safety, including the incidence of dose-limiting toxicities. Efficacy end points included overall response rate, progression-free survival, and overall survival.
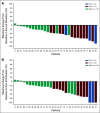
Results: Among the 30 treated patients, dose-limiting toxicities occurred in five patients and included serous retinopathy (n = 2), reversible decreased left ventricular ejection fraction (n = 1), and cetuximab-related infusion reactions (n = 2). The most common grade 3 or 4 adverse events were fatigue (13%), anemia (10%), increased creatine phosphokinase (10%), increased AST (10%), and urinary tract infections (10%). In 29 patients with BRAF V600E-mutant tumors (one patient had a non-BRAF V600E-mutant tumor and was not included in the efficacy analysis), the confirmed overall response rate was 48% (95% CI, 29.4% to 67.5%), median progression-free survival was 8.0 months (95% CI, 5.6 to 9.3 months), and median overall survival was 15.3 months (95% CI, 9.6 months to not reached), with median duration of follow-up of 18.2 months (range, 16.6 to 19.8 months).
Conclusion: In the safety lead-in, the safety and tolerability of the encorafenib, binimetinib, and cetuximab regimen is manageable and acceptable for initiation of the randomized portion of the study. The observed efficacy is promising compared with available therapies and, if confirmed in the randomized portion of the trial, could establish this regimen as a new standard of care for previously treated BRAF V600E-mutant mCRC.
Figures

Comment in
-
The evolving treatment paradigm for BRAF V600 mutant colorectal cancer.Ann Transl Med. 2019 Dec;7(Suppl 8):S257. doi: 10.21037/atm.2019.12.61. Ann Transl Med. 2019. PMID: 32015976 Free PMC article. No abstract available.
-
BRAF mutant metastatic colorectal cancers: new arrows in our quiver.Ann Transl Med. 2019 Dec;7(Suppl 8):S367. doi: 10.21037/atm.2019.08.118. Ann Transl Med. 2019. PMID: 32016085 Free PMC article. No abstract available.
-
Treating BRAFV600E metastatic colorectal patients in 2019: a BEACON of hope?Ann Transl Med. 2019 Nov;7(21):601. doi: 10.21037/atm.2019.08.110. Ann Transl Med. 2019. PMID: 32047762 Free PMC article. No abstract available.
References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084. - PubMed
-
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

