Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study
- PMID: 30892988
- PMCID: PMC6524989
- DOI: 10.1200/JCO.18.01600
Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study
Abstract
Purpose: Effective treatment options are limited for patients with acute myeloid leukemia (AML) who cannot tolerate intensive chemotherapy. An international phase Ib/II study evaluated the safety and preliminary efficacy of venetoclax, a selective B-cell leukemia/lymphoma-2 inhibitor, together with low-dose cytarabine (LDAC) in older adults with AML.
Patients and methods: Adults 60 years or older with previously untreated AML ineligible for intensive chemotherapy were enrolled. Prior treatment of myelodysplastic syndrome, including hypomethylating agents (HMA), was permitted. Eighty-two patients were treated at the recommended phase II dose: venetoclax 600 mg per day orally in 28-day cycles, with LDAC (20 mg/m2 per day) administered subcutaneously on days 1 to 10. Key end points were tolerability, safety, response rates, duration of response (DOR), and overall survival (OS).
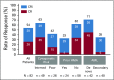
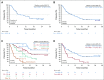
Results: Median age was 74 years (range, 63 to 90 years), 49% had secondary AML, 29% had prior HMA treatment, and 32% had poor-risk cytogenetic features. Common grade 3 or greater adverse events were febrile neutropenia (42%), thrombocytopenia (38%), and WBC count decreased (34%). Early (30-day) mortality was 6%. Fifty-four percent achieved complete remission (CR)/CR with incomplete blood count recovery (median time to first response, 1.4 months). The median OS was 10.1 months (95% CI, 5.7 to 14.2), and median DOR was 8.1 months (95% CI, 5.3 to 14.9 months). Among patients without prior HMA exposure, CR/CR with incomplete blood count recovery was achieved in 62%, median DOR was 14.8 months (95% CI, 5.5 months to not reached), and median OS was 13.5 months (95% CI, 7.0 to 18.4 months).
Conclusion: Venetoclax plus LDAC has a manageable safety profile, producing rapid and durable remissions in older adults with AML ineligible for intensive chemotherapy. High remission rate and low early mortality combined with rapid and durable remission make venetoclax and LDAC an attractive and novel treatment for older adults not suitable for intensive chemotherapy.
Trial registration: ClinicalTrials.gov NCT02287233.
Figures
Comment in
-
Harnessing the Therapeutic Value of Venetoclax: A Breakthrough Therapy in Acute Myeloid Leukemia.J Clin Oncol. 2021 Sep 1;39(25):2742-2748. doi: 10.1200/JCO.21.00080. Epub 2021 Jun 4. J Clin Oncol. 2021. PMID: 34086506 Free PMC article. No abstract available.
References
-
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. - PubMed
-
- US Department of Health and Human Services, National Institutes of Health. National Cancer Institute: Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts: Leukemia – Acute Myeloid Leukemia (AML). 2018 https://seer.cancer.gov/statfacts/html/amyl.html.
-
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. - PubMed
-
- Dennis M, Hills RK, Russell NH, et al: An evaluation of 17 years of low dose cytarabine as therapy for AML patients not fit for intensive treatment, including patients with adverse cytogenetics, shows improving survival, potential underutilisation and highlights the need for new therapy. Blood 2017; 130:3874.
-
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

