Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice
- PMID: 30893295
- PMCID: PMC6443191
- DOI: 10.1371/journal.pbio.3000189
Ablating astrocyte insulin receptors leads to delayed puberty and hypogonadism in mice
Abstract
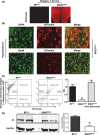
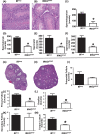
Insulin resistance and obesity are associated with reduced gonadotropin-releasing hormone (GnRH) release and infertility. Mice that lack insulin receptors (IRs) throughout development in both neuronal and non-neuronal brain cells are known to exhibit subfertility due to hypogonadotropic hypogonadism. However, attempts to recapitulate this phenotype by targeting specific neurons have failed. To determine whether astrocytic insulin sensing plays a role in the regulation of fertility, we generated mice lacking IRs in astrocytes (astrocyte-specific insulin receptor deletion [IRKOGFAP] mice). IRKOGFAP males and females showed a delay in balanopreputial separation or vaginal opening and first estrous, respectively. In adulthood, IRKOGFAP female mice also exhibited longer, irregular estrus cycles, decreased pregnancy rates, and reduced litter sizes. IRKOGFAP mice show normal sexual behavior but hypothalamic-pituitary-gonadotropin (HPG) axis dysregulation, likely explaining their low fecundity. Histological examination of testes and ovaries showed impaired spermatogenesis and ovarian follicle maturation. Finally, reduced prostaglandin E synthase 2 (PGES2) levels were found in astrocytes isolated from these mice, suggesting a mechanism for low GnRH/luteinizing hormone (LH) secretion. These findings demonstrate that insulin sensing by astrocytes is indispensable for the function of the reproductive axis. Additional work is needed to elucidate the role of astrocytes in the maturation of hypothalamic reproductive circuits.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Gonadotropin-Releasing Hormone Receptor (GnRHR) and Hypogonadotropic Hypogonadism.Int J Mol Sci. 2023 Nov 4;24(21):15965. doi: 10.3390/ijms242115965. Int J Mol Sci. 2023. PMID: 37958948 Free PMC article. Review.
-
Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells.Endocrinology. 2013 Mar;154(3):1337-48. doi: 10.1210/en.2012-2056. Epub 2013 Feb 7. Endocrinology. 2013. PMID: 23392256 Free PMC article.
-
Deletion of Vax1 from Gonadotropin-Releasing Hormone (GnRH) Neurons Abolishes GnRH Expression and Leads to Hypogonadism and Infertility.J Neurosci. 2016 Mar 23;36(12):3506-18. doi: 10.1523/JNEUROSCI.2723-15.2016. J Neurosci. 2016. PMID: 27013679 Free PMC article.
-
Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes.J Neurosci. 2003 Jan 1;23(1):230-9. doi: 10.1523/JNEUROSCI.23-01-00230.2003. J Neurosci. 2003. PMID: 12514220 Free PMC article.
-
The role of non-neuronal cells in hypogonadotropic hypogonadism.Mol Cell Endocrinol. 2020 Dec 1;518:110996. doi: 10.1016/j.mce.2020.110996. Epub 2020 Aug 26. Mol Cell Endocrinol. 2020. PMID: 32860862 Review.
Cited by
-
Insulin action in the brain: cell types, circuits, and diseases.Trends Neurosci. 2022 May;45(5):384-400. doi: 10.1016/j.tins.2022.03.001. Epub 2022 Mar 28. Trends Neurosci. 2022. PMID: 35361499 Free PMC article. Review.
-
Peripheral Insulin Regulates a Broad Network of Gene Expression in Hypothalamus, Hippocampus, and Nucleus Accumbens.Diabetes. 2021 Aug;70(8):1857-1873. doi: 10.2337/db20-1119. Epub 2021 May 24. Diabetes. 2021. PMID: 34031123 Free PMC article.
-
The Significance of Hypothalamic Inflammation and Gliosis for the Pathogenesis of Obesity in Humans.Endocr Rev. 2023 Mar 4;44(2):281-296. doi: 10.1210/endrev/bnac023. Endocr Rev. 2023. PMID: 36251886 Free PMC article. Review.
-
Insulin sensing by astrocytes is critical for normal thermogenesis and body temperature regulation.J Endocrinol. 2020 Oct;247(1):39-52. doi: 10.1530/JOE-20-0052. J Endocrinol. 2020. PMID: 32698146 Free PMC article.
-
Pituitary P62 deficiency leads to female infertility by impairing luteinizing hormone production.Exp Mol Med. 2021 Aug;53(8):1238-1249. doi: 10.1038/s12276-021-00661-4. Epub 2021 Aug 27. Exp Mol Med. 2021. PMID: 34453106 Free PMC article.
References
-
- Griffin ML, South SA, Yankov VI, Booth RA Jr., Asplin CM, Veldhuis JD, et al. Insulin-dependent diabetes mellitus and menstrual dysfunction. Annals of medicine. 1994;26(5):331–40. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

