Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins
- PMID: 30893348
- PMCID: PMC6426197
- DOI: 10.1371/journal.pone.0213346
Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins
Abstract
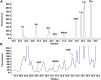
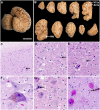
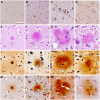
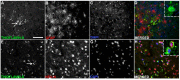
Dolphin stranding events occur frequently in Florida and Massachusetts. Dolphins are an excellent sentinel species for toxin exposures in the marine environment. In this report we examine whether cyanobacterial neurotoxin, β-methylamino-L-alanine (BMAA), is present in stranded dolphins. BMAA has been shown to bioaccumulate in the marine food web, including in the muscles and fins of sharks. Dietary exposure to BMAA is associated with the occurrence of neurofibrillary tangles and β-amyloid plaques in nonhuman primates. The findings of protein-bound BMAA in brain tissues from patients with Alzheimer's disease has advanced the hypothesis that BMAA may be linked to dementia. Since dolphins are apex predators and consume prey containing high amounts of BMAA, we examined necropsy specimens to determine if dietary and environmental exposures may result in the accumulation of BMAA in the brains of dolphins. To test this hypothesis, we measured BMAA in a series of brains collected from dolphins stranded in Florida and Massachusetts using two orthogonal analytical methods: 1) high performance liquid chromatography, and 2) ultra-performance liquid chromatography with tandem mass spectrometry. We detected high levels of BMAA (20-748 μg/g) in the brains of 13 of 14 dolphins. To correlate neuropathological changes with toxin exposure, gross and microscopic examinations were performed on cortical brain regions responsible for acoustico-motor navigation. We observed increased numbers of β-amyloid+ plaques and dystrophic neurites in the auditory cortex compared to the visual cortex and brainstem. The presence of BMAA and neuropathological changes in the stranded dolphin brain may help to further our understanding of cyanotoxin exposure and its potential impact on human health.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Environmental neurotoxins β-N-methylamino-l-alanine (BMAA) and mercury in shark cartilage dietary supplements.Food Chem Toxicol. 2014 Aug;70:26-32. doi: 10.1016/j.fct.2014.04.015. Epub 2014 Apr 19. Food Chem Toxicol. 2014. PMID: 24755394
-
BMAA, Methylmercury, and Mechanisms of Neurodegeneration in Dolphins: A Natural Model of Toxin Exposure.Toxins (Basel). 2021 Oct 1;13(10):697. doi: 10.3390/toxins13100697. Toxins (Basel). 2021. PMID: 34678990 Free PMC article.
-
Cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) in shark fins.Mar Drugs. 2012 Feb;10(2):509-520. doi: 10.3390/md10020509. Epub 2012 Feb 21. Mar Drugs. 2012. PMID: 22412816 Free PMC article.
-
The Cyanotoxin and Non-protein Amino Acid β-Methylamino-L-Alanine (L-BMAA) in the Food Chain: Incorporation into Proteins and Its Impact on Human Health.Neurotox Res. 2019 Oct;36(3):602-611. doi: 10.1007/s12640-019-00089-9. Epub 2019 Aug 3. Neurotox Res. 2019. PMID: 31377995 Review.
-
Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity.Neurotox Res. 2018 Jan;33(1):168-177. doi: 10.1007/s12640-017-9757-2. Epub 2017 Jun 5. Neurotox Res. 2018. PMID: 28585115 Review.
Cited by
-
Editorial: New insights in the neuroanatomy and neuropathology of marine mammals.Front Neuroanat. 2024 Jul 1;18:1449199. doi: 10.3389/fnana.2024.1449199. eCollection 2024. Front Neuroanat. 2024. PMID: 39011054 Free PMC article. No abstract available.
-
Methodology and Neuromarkers for Cetaceans' Brains.Vet Sci. 2022 Jan 21;9(2):38. doi: 10.3390/vetsci9020038. Vet Sci. 2022. PMID: 35202291 Free PMC article.
-
The Effects of Long-term, Low-dose β-N-methylamino-L-alanine (BMAA) Exposures in Adult SODG93R Transgenic Zebrafish.Neurotox Res. 2023 Oct;41(5):481-495. doi: 10.1007/s12640-023-00658-z. Epub 2023 Aug 8. Neurotox Res. 2023. PMID: 37552461 Free PMC article.
-
Cyanotoxins and the Nervous System.Toxins (Basel). 2021 Sep 16;13(9):660. doi: 10.3390/toxins13090660. Toxins (Basel). 2021. PMID: 34564664 Free PMC article. Review.
-
Central Nervous System Disorders of Marine Mammals: Models for Human Disease?Pathogens. 2024 Aug 14;13(8):684. doi: 10.3390/pathogens13080684. Pathogens. 2024. PMID: 39204284 Free PMC article. Review.
References
-
- Lapointe BE, Tomasko DA, and Matzie WR (1994) Eutrophication and trophic state classification of seagrass communities in the Florida Keys. Bulletin of Marine Science 54: 696–717.
-
- Morabito S, Silvestro S, Faggio C (2017) How the marine biotoxins affect human health. Nat Prod Res: 1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

