Sympatric versus allopatric evolutionary contexts shape differential immune response in Biomphalaria / Schistosoma interaction
- PMID: 30893368
- PMCID: PMC6443186
- DOI: 10.1371/journal.ppat.1007647
Sympatric versus allopatric evolutionary contexts shape differential immune response in Biomphalaria / Schistosoma interaction
Abstract
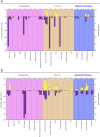
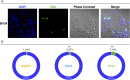
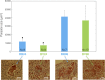
Selective pressures between hosts and their parasites can result in reciprocal evolution or adaptation of specific life history traits. Local adaptation of resident hosts and parasites should lead to increase parasite infectivity/virulence (higher compatibility) when infecting hosts from the same location (in sympatry) than from a foreign location (in allopatry). Analysis of geographic variations in compatibility phenotypes is the most common proxy used to infer local adaptation. However, in some cases, allopatric host-parasite systems demonstrate similar or greater compatibility than in sympatry. In such cases, the potential for local adaptation remains unclear. Here, we study the interaction between Schistosoma and its vector snail Biomphalaria in which such discrepancy in local versus foreign compatibility phenotype has been reported. Herein, we aim at bridging this gap of knowledge by comparing life history traits (immune cellular response, host mortality, and parasite growth) and molecular responses in highly compatible sympatric and allopatric Schistosoma/Biomphalaria interactions originating from different geographic localities (Brazil, Venezuela and Burundi). We found that despite displaying similar prevalence phenotypes, sympatric schistosomes triggered a rapid immune suppression (dual-RNAseq analyses) in the snails within 24h post infection, whereas infection by allopatric schistosomes (regardless of the species) was associated with immune cell proliferation and triggered a non-specific generalized immune response after 96h. We observed that, sympatric schistosomes grow more rapidly. Finally, we identify miRNAs differentially expressed by Schistosoma mansoni that target host immune genes and could be responsible for hijacking the host immune response during the sympatric interaction. We show that despite having similar prevalence phenotypes, sympatric and allopatric snail-Schistosoma interactions displayed strong differences in their immunobiological molecular dialogue. Understanding the mechanisms allowing parasites to adapt rapidly and efficiently to new hosts is critical to control disease emergence and risks of Schistosomiasis outbreaks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. Schistosomiasis Fact Sheet No 115 World Health Organisation; 2010.
-
- Basch PF. Intermediate host specificity in Schistosoma mansoni. Experimental Parasitology. 1976;39(1):150–69. - PubMed
-
- Theron A, Rognon A, Gourbal B, Mitta G. Multi-parasite host susceptibility and multi-host parasite infectivity: a new approach of the Biomphalaria glabrata/Schistosoma mansoni compatibility polymorphism. Infection Genetics and Evolution. 2014;26:80–8. - PubMed
-
- Prugnolle F, de Meeus T, Pointier JP, Durand P, Rognon A, Theron A. Geographical variations in infectivity and susceptibility in the host-parasite system Schistosoma mansoni/Biomphalaria glabrata: no evidence for local adaptation. Parasitology. 2006;133(Pt 3):313–9. 10.1017/S0031182006000412 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

