Bacterial fitness in chronic wounds appears to be mediated by the capacity for high-density growth, not virulence or biofilm functions
- PMID: 30893371
- PMCID: PMC6448920
- DOI: 10.1371/journal.ppat.1007511
Bacterial fitness in chronic wounds appears to be mediated by the capacity for high-density growth, not virulence or biofilm functions
Abstract
While much is known about acute infection pathogenesis, the understanding of chronic infections has lagged. Here we sought to identify the genes and functions that mediate fitness of the pathogen Pseudomonas aeruginosa in chronic wound infections, and to better understand the selective environment in wounds. We found that clinical isolates from chronic human wounds were frequently defective in virulence functions and biofilm formation, and that many virulence and biofilm formation genes were not required for bacterial fitness in experimental mouse wounds. In contrast, genes involved in anaerobic growth, some metabolic and energy pathways, and membrane integrity were critical. Consistent with these findings, the fitness characteristics of some wound impaired-mutants could be represented by anaerobic, oxidative, and membrane-stress conditions ex vivo, and more comprehensively by high-density bacterial growth conditions, in the absence of a host. These data shed light on the bacterial functions needed in chronic wound infections, the nature of stresses applied to bacteria at chronic infection sites, and suggest therapeutic targets that might compromise wound infection pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
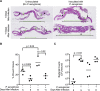
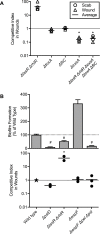




References
-
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2(9):1051–60. Epub 2000/09/01. S1286-4579(00)01259-4 [pii]. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

