Review of disease-related complications and management in adult patients with thalassemia: A multi-center study in Thailand
- PMID: 30893381
- PMCID: PMC6426207
- DOI: 10.1371/journal.pone.0214148
Review of disease-related complications and management in adult patients with thalassemia: A multi-center study in Thailand
Abstract
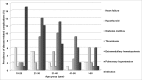
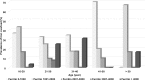
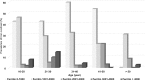
Disease-related complications and management are different among patients with thalassemia. This study was aimed to review the prevalence, clinical risk factors for the complications and the management in patients with thalassemia in Thailand. A multicenter cross-sectional study was conducted in patients with thalassemia aged ≥ 18 years old. Thalassemia-related complications and management were reviewed. The clinical parameters significantly associated with the complications were analyzed by logistic regression methods. The prevalence of thalassemia-related complications was 100% in patients with transfusion-dependent thalassemia (TDT) and 58.8% in patients with non-transfusion-dependent thalassemia (NTDT). Advanced age was statistically associated with extramedullary hematopoiesis in both TDT and NTDT patients. Splenectomy was a significant risk factor for pulmonary hypertension in both groups of patients. Severe iron overload started earlier in patients with TDT than NTDT and was associated with diabetes mellitus (adjusted odds ratio (AOR) = 6.2, p-value = 0.02). Disease-related complications are more prevalent in patients with TDT than patients with NTDT. Splenectomy and advanced age were important risk factors for developing major complications in both groups. Early screening and management for specific disease-related complications should be considered in patients with thalassemia according to their clinical risk factors.
Conflict of interest statement
No Novartis or any pharmaceutical company products nor honoraria were used to conduct any of the research as part of this publication. Chuncharunee S has received research funding and honoraria from Novartis Pharmaceuticals, Janssen-Cilag, Roche, and Celgene. Teawtrakul N received honoraria from Novartis and Janssen-Cilag. Siritanaratkul N. received research funding and honoraria from Novartis, Janssen-Cilag, Roche, and Pfizer. The remaining author declares no competing financial interests. There are no patents, products in development or marketed products to declare. This does not alter our adherence to the PLOS ONE policies on sharing data and materials.
Figures
References
-
- Thein SL. Genetic modifiers of beta-thalassemia. Haematologica. 2005;90:649–60. - PubMed
-
- Thein SL. The emerging role of fetal hemoglobin induction in non-transfusion-dependent thalassemia. Blood Rev. 2012;26 Suppl 1:S35–39. - PubMed
-
- Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev. 2012;26 Suppl 1:S3–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

