Psychosis with Methylphenidate or Amphetamine in Patients with ADHD
- PMID: 30893533
- PMCID: PMC6543546
- DOI: 10.1056/NEJMoa1813751
Psychosis with Methylphenidate or Amphetamine in Patients with ADHD
Abstract
Background: The prescription use of the stimulants methylphenidate and amphetamine for the treatment of attention deficit-hyperactivity disorder (ADHD) has been increasing. In 2007, the Food and Drug Administration mandated changes to drug labels for stimulants on the basis of findings of new-onset psychosis. Whether the risk of psychosis in adolescents and young adults with ADHD differs among various stimulants has not been extensively studied.
Methods: We used data from two commercial insurance claims databases to assess patients 13 to 25 years of age who had received a diagnosis of ADHD and who started taking methylphenidate or amphetamine between January 1, 2004, and September 30, 2015. The outcome was a new diagnosis of psychosis for which an antipsychotic medication was prescribed during the first 60 days after the date of the onset of psychosis. To estimate hazard ratios for psychosis, we used propensity scores to match patients who received methylphenidate with patients who received amphetamine in each database, compared the incidence of psychosis between the two stimulant groups, and then pooled the results across the two databases.
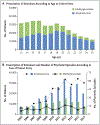
Results: We assessed 337,919 adolescents and young adults who received a prescription for a stimulant for ADHD. The study population consisted of 221,846 patients with 143,286 person-years of follow up; 110,923 patients taking methylphenidate were matched with 110,923 patients taking amphetamines. There were 343 episodes of psychosis (with an episode defined as a new diagnosis code for psychosis and a prescription for an antipsychotic medication) in the matched populations (2.4 per 1000 person-years): 106 episodes (0.10%) in the methylphenidate group and 237 episodes (0.21%) in the amphetamine group (hazard ratio with amphetamine use, 1.65; 95% confidence interval, 1.31 to 2.09).
Conclusions: Among adolescents and young adults with ADHD who were receiving prescription stimulants, new-onset psychosis occurred in approximately 1 in 660 patients. Amphetamine use was associated with a greater risk of psychosis than methylphenidate. (Funded by the National Institute of Mental Health and others.).
Copyright © 2019 Massachusetts Medical Society.
Figures


Comment in
-
Psychosis during Attention Deficit-Hyperactivity Disorder Treatment with Stimulants.N Engl J Med. 2019 Mar 21;380(12):1178-1180. doi: 10.1056/NEJMe1900502. N Engl J Med. 2019. PMID: 30893541 No abstract available.
References
-
- Jensen PS, Hinshaw SP, Swanson JM, et al. Findings from the NIMH Multi-modal Treatment Study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr 2001; 22: 60–73. - PubMed
-
- Olfson M, Blanco C, Wang S, Green-hill LL. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry 2013; 74: 43–50. - PubMed
-
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011; 128: 1007–22. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
