The Dual Prey-Inactivation Strategy of Spiders-In-Depth Venomic Analysis of Cupiennius salei
- PMID: 30893800
- PMCID: PMC6468893
- DOI: 10.3390/toxins11030167
The Dual Prey-Inactivation Strategy of Spiders-In-Depth Venomic Analysis of Cupiennius salei
Abstract
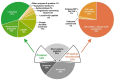
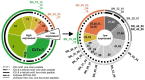
Most knowledge of spider venom concerns neurotoxins acting on ion channels, whereas proteins and their significance for the envenomation process are neglected. The here presented comprehensive analysis of the venom gland transcriptome and proteome of Cupiennius salei focusses on proteins and cysteine-containing peptides and offers new insight into the structure and function of spider venom, here described as the dual prey-inactivation strategy. After venom injection, many enzymes and proteins, dominated by α-amylase, angiotensin-converting enzyme, and cysteine-rich secretory proteins, interact with main metabolic pathways, leading to a major disturbance of the cellular homeostasis. Hyaluronidase and cytolytic peptides destroy tissue and membranes, thus supporting the spread of other venom compounds. We detected 81 transcripts of neurotoxins from 13 peptide families, whereof two families comprise 93.7% of all cysteine-containing peptides. This raises the question of the importance of the other low-expressed peptide families. The identification of a venom gland-specific defensin-like peptide and an aga-toxin-like peptide in the hemocytes offers an important clue on the recruitment and neofunctionalization of body proteins and peptides as the origin of toxins.
Keywords: enzymes; in depth transcriptomics; neurotoxins; proteomics; venom; α-amylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Proteotranscriptomic Insights into the Venom Composition of the Wolf Spider Lycosa tarantula.Toxins (Basel). 2020 Aug 5;12(8):501. doi: 10.3390/toxins12080501. Toxins (Basel). 2020. PMID: 32764230 Free PMC article.
-
Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae).Toxicon. 2004 Apr;43(5):543-53. doi: 10.1016/j.toxicon.2004.02.009. Toxicon. 2004. PMID: 15066412 Review.
-
Isolation, N-glycosylations and Function of a Hyaluronidase-Like Enzyme from the Venom of the Spider Cupiennius salei.PLoS One. 2015 Dec 2;10(12):e0143963. doi: 10.1371/journal.pone.0143963. eCollection 2015. PLoS One. 2015. PMID: 26630650 Free PMC article.
-
Complex precursor structures of cytolytic cupiennins identified in spider venom gland transcriptomes.Sci Rep. 2021 Feb 17;11(1):4009. doi: 10.1038/s41598-021-83624-z. Sci Rep. 2021. PMID: 33597701 Free PMC article.
-
Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses.Toxins (Basel). 2019 Oct 22;11(10):611. doi: 10.3390/toxins11100611. Toxins (Basel). 2019. PMID: 31652611 Free PMC article. Review.
Cited by
-
A Long-Read Genome Assembly of a Native Mite in China Pyemotes zhonghuajia Yu, Zhang & He (Prostigmata: Pyemotidae) Reveals Gene Expansion in Toxin-Related Gene Families.Toxins (Basel). 2022 Aug 21;14(8):571. doi: 10.3390/toxins14080571. Toxins (Basel). 2022. PMID: 36006233 Free PMC article.
-
Venomics Approach Reveals a High Proportion of Lactrodectus-Like Toxins in the Venom of the Noble False Widow Spider Steatoda nobilis.Toxins (Basel). 2020 Jun 18;12(6):402. doi: 10.3390/toxins12060402. Toxins (Basel). 2020. PMID: 32570718 Free PMC article.
-
An Economic Dilemma Between Molecular Weapon Systems May Explain an Arachno-atypical Venom in Wasp Spiders (Argiope bruennichi).Biomolecules. 2020 Jun 30;10(7):978. doi: 10.3390/biom10070978. Biomolecules. 2020. PMID: 32630016 Free PMC article.
-
A Multiomics Approach Unravels New Toxins With Possible In Silico Antimicrobial, Antiviral, and Antitumoral Activities in the Venom of Acanthoscurria rondoniae.Front Pharmacol. 2020 Jul 17;11:1075. doi: 10.3389/fphar.2020.01075. eCollection 2020. Front Pharmacol. 2020. PMID: 32774304 Free PMC article.
-
Transcriptome-Wide Prediction and Measurement of Combined Effects Induced by Chemical Mixture Exposure in Zebrafish Embryos.Environ Health Perspect. 2021 Apr;129(4):47006. doi: 10.1289/EHP7773. Epub 2021 Apr 7. Environ Health Perspect. 2021. PMID: 33826412 Free PMC article.
References
-
- Natural History Museum Bern; [(accessed on 11 September 2018)]. World Spider Catalog. Version 19.5. Available online: http://wsc.nmbe.ch.
-
- Wise D.H. Spiders in Ecological Webs. Cambridge University Press; Cambridge, MA, USA: 1993.
-
- Kuhn-Nentwig L., Stöcklin R., Nentwig W. Venom composition and strategies in spiders: Is everything possible? Adv. Insect Physiol. 2011;40:1–86.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

