Calcium Carbonate Nanoparticles Can Activate the Epithelial⁻Mesenchymal Transition in an Experimental Gastric Cancer Model
- PMID: 30893803
- PMCID: PMC6466388
- DOI: 10.3390/biomedicines7010021
Calcium Carbonate Nanoparticles Can Activate the Epithelial⁻Mesenchymal Transition in an Experimental Gastric Cancer Model
Abstract
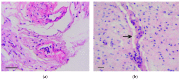
Previously, we have shown the possibility of intramucosal gastric carcinoma induction by the intragastric administration of a mixture of formaldehyde and hydrogen peroxide in rats. In this study, we report a sizable increase in carcinogenic properties of the mixture when a suspension containing calcium carbonate nanoparticles was added to it. This technique allowed us to reduce both the number of the carcinogen administrations from twelve to two and the time to the cancer induction from six to four months. Although the induced tumors were represented by the intramucosal carcinomas, they were characterized by the extensive invasion of individual tumor cells and their clusters into the muscle layer and serosa as well as into the omentum and blood vessels. Considering that the invasive tumor cells were positive for vimentin, Snail and TGF-β2, we concluded that their invasion was the result of the activation of epithelial⁻mesenchymal transition (EMT) mechanisms. Thus, taking into account the data obtained, it can be assumed that under the conditions of inflammation or carcinogenesis, the calcium carbonate nanoparticles may affect the activation of EMT mechanisms.
Keywords: calcium carbonate; carcinogenesis; epithelial–mesenchymal transition; gastric cancer; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Chen X.L., Zhao L.Y., Xue L., Xu Y.H., Zhang W.H., Liu K., Chen X.Z., Yang K., Zhang B., Chen Z.X., et al. Prognostic significance and the role in TNM stage of extranodal metastasis within regional lymph nodes station in gastric carcinoma. Oncotarget. 2016;7:67047–67060. doi: 10.18632/oncotarget.11478. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

