Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis
- PMID: 30893856
- PMCID: PMC6471475
- DOI: 10.3390/ijms20061383
Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis
Abstract
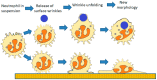
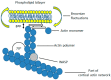
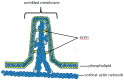
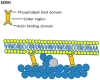
Perhaps the most important feature of neutrophils is their ability to rapidly change shape. In the bloodstream, the neutrophils circulate as almost spherical cells, with the ability to deform in order to pass along narrower capillaries. Upon receiving the signal to extravasate, they are able to transform their morphology and flatten onto the endothelium surface. This transition, from a spherical to a flattened morphology, is the first key step which neutrophils undergo before moving out of the blood and into the extravascular tissue space. Once they have migrated through tissues towards sites of infection, neutrophils carry out their primary role-killing infecting microbes by performing phagocytosis and producing toxic reactive oxygen species within the microbe-containing phagosome. Phagocytosis involves the second key morphology change that neutrophils undergo, with the formation of pseudopodia which capture the microbe within an internal vesicle. Both the spherical to flattened stage and the phagocytic capture stage are rapid, each being completed within 100 s. Knowing how these rapid cell shape changes occur in neutrophils is thus fundamental to understanding neutrophil behaviour. This article will discuss advances in our current knowledge of this process, and also identify an important regulated molecular event which may represent an important target for anti-inflammatory therapy.
Keywords: Ca2+; calpain; cell spreading; cortical actin; ezrin; membrane expansion; membrane tension; neutrophils; phagocytosis.
Conflict of interest statement
There are no potential conflicts of interest.
Figures


Similar articles
-
Ca²⁺ and calpain control membrane expansion during the rapid cell spreading of neutrophils.J Cell Sci. 2013 Oct 15;126(Pt 20):4627-35. doi: 10.1242/jcs.124917. Epub 2013 Aug 13. J Cell Sci. 2013. PMID: 23943875 Free PMC article.
-
Phagocytosis and Motility in Human Neutrophils is Competent but Compromised by Pharmacological Inhibition of Ezrin Phosphorylation.Curr Mol Pharmacol. 2018;11(4):305-315. doi: 10.2174/1874467211666180516100613. Curr Mol Pharmacol. 2018. PMID: 29766831
-
Cell surface topography controls phagocytosis and cell spreading: The membrane reservoir in neutrophils.Biochim Biophys Acta Mol Cell Res. 2020 Dec;1867(12):118832. doi: 10.1016/j.bbamcr.2020.118832. Epub 2020 Aug 27. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32860836
-
Membrane Tension and the Role of Ezrin During Phagocytosis.Adv Exp Med Biol. 2020;1246:83-102. doi: 10.1007/978-3-030-40406-2_6. Adv Exp Med Biol. 2020. PMID: 32399827 Review.
-
Calpain Activation by Ca2+ and Its Role in Phagocytosis.Adv Exp Med Biol. 2020;1246:129-151. doi: 10.1007/978-3-030-40406-2_8. Adv Exp Med Biol. 2020. PMID: 32399829 Review.
Cited by
-
Phagocyte Transcriptomic Analysis Reveals Focal Adhesion Kinase (FAK) and Heparan Sulfate Proteoglycans (HSPGs) as Major Regulators in Anti-bacterial Defense of Crassostrea hongkongensis.Front Immunol. 2020 Mar 20;11:416. doi: 10.3389/fimmu.2020.00416. eCollection 2020. Front Immunol. 2020. PMID: 32265912 Free PMC article.
-
Structure, Biosynthesis, and Biological Activity of Succinylated Forms of Bacteriocin BacSp222.Int J Mol Sci. 2021 Jun 10;22(12):6256. doi: 10.3390/ijms22126256. Int J Mol Sci. 2021. PMID: 34200765 Free PMC article.
-
[Re-understanding the physiological and pathophysiological roles of neutrophils].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Feb 20;38(2):109-113. doi: 10.3760/cma.j.cn501120-20211122-00391. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35220698 Free PMC article. Chinese.
-
Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils.Cancers (Basel). 2022 Jul 11;14(14):3366. doi: 10.3390/cancers14143366. Cancers (Basel). 2022. PMID: 35884427 Free PMC article. Review.
-
Recent Advances in Membrane-Coated Micro/Nanomotors in Biological Applications.Int J Nanomedicine. 2025 Jul 28;20:9427-9446. doi: 10.2147/IJN.S526671. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40755462 Free PMC article. Review.
References
-
- Bessis M. Living Blood Cells and Their Ultrastructure. Springer; Berlin, Germany: 1973.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

