BACE-1 and γ-Secretase as Therapeutic Targets for Alzheimer's Disease
- PMID: 30893882
- PMCID: PMC6469197
- DOI: 10.3390/ph12010041
BACE-1 and γ-Secretase as Therapeutic Targets for Alzheimer's Disease
Abstract
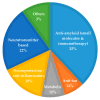
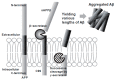
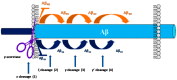
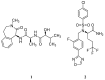
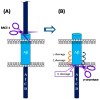
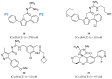
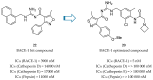
Alzheimer's disease (AD) is a growing global health concern with a massive impact on affected individuals and society. Despite the considerable advances achieved in the understanding of AD pathogenesis, researchers have not been successful in fully identifying the mechanisms involved in disease progression. The amyloid hypothesis, currently the prevalent theory for AD, defends the deposition of β-amyloid protein (Aβ) aggregates as the trigger of a series of events leading to neuronal dysfunction and dementia. Hence, several research and development (R&D) programs have been led by the pharmaceutical industry in an effort to discover effective and safety anti-amyloid agents as disease modifying agents for AD. Among 19 drug candidates identified in the AD pipeline, nine have their mechanism of action centered in the activity of β or γ-secretase proteases, covering almost 50% of the identified agents. These drug candidates must fulfill the general rigid prerequisites for a drug aimed for central nervous system (CNS) penetration and selectivity toward different aspartyl proteases. This review presents the classes of γ-secretase and beta-site APP cleaving enzyme 1 (BACE-1) inhibitors under development, highlighting their structure-activity relationship, among other physical-chemistry aspects important for the successful development of new anti-AD pharmacological agents.
Keywords: Alzheimer’s disease; BACE-1; amyloid hypothesis; γ-secretase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


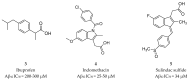
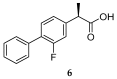
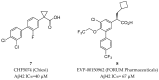
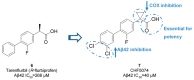
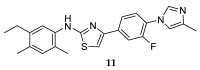
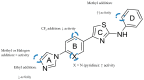
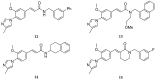
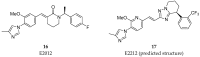
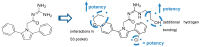
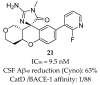
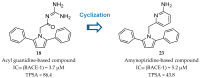
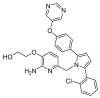
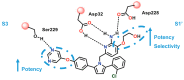
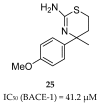
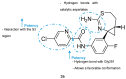
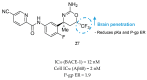
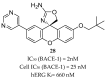
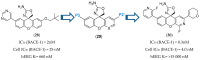
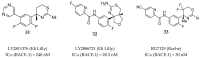
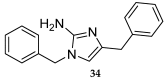
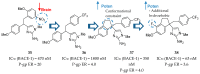
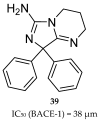
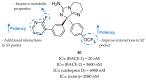
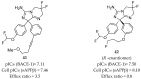
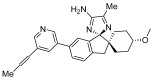

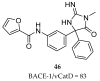
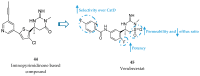
References
-
- Duthey B. Background Paper 6.11 Alzheimer Disease and Other Dementias, Update on 2004. World Health Organization; Geneva, Switzerland: Feb, 2013. pp. 1–77.
-
- Eurostat Population Structure and Ageing. [(accessed on 5 January 2019)]; Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_s....
-
- Alzheimer’s Association 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017;13:325–373.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

