Radiosensitivity of Cancer Cells Is Regulated by Translationally Controlled Tumor Protein
- PMID: 30893896
- PMCID: PMC6468585
- DOI: 10.3390/cancers11030386
Radiosensitivity of Cancer Cells Is Regulated by Translationally Controlled Tumor Protein
Abstract
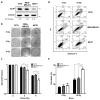
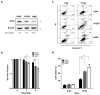
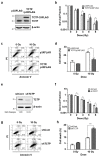
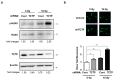
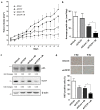
Translationally controlled tumor protein (TCTP) is a ubiquitous multifunctional protein that is essential for cell survival. This study reveals that the regulation of radiosensitivity of cancer cells is yet another function of TCTP. The relationship between endogenous TCTP levels and sensitivity to radiation was examined in breast cancer cell lines (T47D, MDA-MB-231, and MCF7) and lung cancer cells lines (A549, H1299, and H460). Cancer cells with high expression levels of TCTP were more resistant to radiation. TCTP overexpression inhibited radiation-induced cell death, while silencing TCTP led to an increase in radiosensitivity. DNA damage in the irradiated TCTP-silenced A549 cells was greater than in irradiated control shRNA-transfected A549 cells. p53, a well-known reciprocal regulator of TCTP, was increased in irradiated TCTP down-regulated A549 cells. Moreover, introduction of p53 siRNA in TCTP knocked-down A549 cells abrogated the increased radiosensitivity induced by TCTP knockdown. An in vivo xenograft study also confirmed enhanced radiosensitivity in TCTP down-regulated A549 cells. These findings suggest that TCTP has the potential to serve as a therapeutic target to overcome radiation resistance in cancer, a major problem for the effective treatment of cancers.
Keywords: TCTP; p53; radioresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Harrington K.J., Billingham L.J., Brunner T.B., Burnet N.G., Chan C.S., Hoskin P., Mackay R.I., Maughan T.S., Macdougall J., McKenna W.G., et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer. 2011;105:628–639. doi: 10.1038/bjc.2011.240. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

