Recent Advances in Phenylboronic Acid-Based Gels with Potential for Self-Regulated Drug Delivery
- PMID: 30893913
- PMCID: PMC6470492
- DOI: 10.3390/molecules24061089
Recent Advances in Phenylboronic Acid-Based Gels with Potential for Self-Regulated Drug Delivery
Abstract
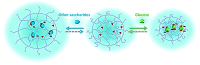
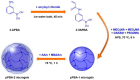
Glucose-sensitive drug platforms are highly attractive in the field of self-regulated drug delivery. Drug carriers based on boronic acid (BA), especially phenylboronic acid (PBA), have been designed for glucose-sensitive self-regulated insulin delivery. The PBA-functionalized gels have attracted more interest in recent years. The cross-linked three-dimensional (3D) structure endows the glucose-sensitive gels with great physicochemical properties. The PBA-based platforms with cross-linked structures have found promising applications in self-regulated drug delivery systems. This article summarizes some recent attempts at the developments of PBA-mediated glucose-sensitive gels for self-regulated drug delivery. The PBA-based glucose-sensitive gels, including hydrogels, microgels, and nanogels, are expected to significantly promote the development of smart self-regulated drug delivery systems for diabetes therapy.
Keywords: diabetes therapy; drug delivery; gel; glucose sensitivity; phenylboronic acid.
Conflict of interest statement
There is no conflict of interest regarding this manuscript.
Figures

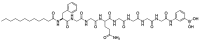


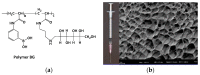





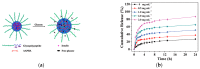
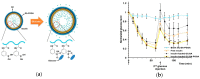

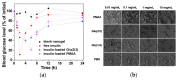
References
-
- Tripathi B.K., Srivastava A.K. Diabetes mellitus: Complications and therapeutics. Med. Sci. Monit. 2006;12:RA130–RA147. - PubMed
-
- Chou D.H.-C., Webber M.J., Tang B.C., Lin A.B., Thapa L.S., Deng D., Truong J.V., Cortinas A.B., Langer R., Anderson D.G. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. USA. 2015;112:2401–2406. doi: 10.1073/pnas.1424684112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

