Transcriptome profiling reveals association of peripheral adipose tissue pathology with type-2 diabetes in Asian Indians
- PMID: 30894049
- PMCID: PMC6768216
- DOI: 10.1080/21623945.2019.1595269
Transcriptome profiling reveals association of peripheral adipose tissue pathology with type-2 diabetes in Asian Indians
Abstract
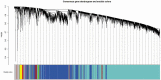
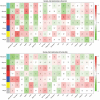
Type 2 diabetes (T2D) is a complex disease with an elusive link between its molecular aetiology and clinical presentation. Although, the role of visceral adipose tissue in insulin-resistance and T2D is known, limited information is available on the role of peripheral-subcutaneous adipose tissue especially in Asian Indians. In this microarray-based study of diabetic and normal glucose tolerant Asian Indians, we generated the transcriptome of their thigh adipose tissue and analyzed differentially expressed genes (DEGs) using weighted gene co-expression network analysis; further we identified perturbed pathways implicated by these DEGs in relevant co-expression modules. We also attempted to link these pathways with known aspects of T2D pathophysiology in terms of their association with some of their intermediate traits, namely; adipocyte size, HOMA-B, HOMA-R, Hb1Ac, insulin, glucose-level, TNF-α, IL-6, VLDLs, LDLs, HDLs, and NEFAs. It was observed that several modules of co-expressed genes show an association with diabetes and some of its intermediate phenotypic traits mentioned above. Therefore, these findings suggest a role of peripheral subcutaneous adipose tissue in the pathophsiology of T2D in Asian Indians. Additionally, our study indicated that the peripheral subcutaneous adipose tissue in diabetics shows pathologic changes characterized by adipocyte hypertrophy and up-regulation of inflammation-related pathways.
Keywords: Asian Indians; Type 2 diabetes; peripheral adipose tissue; signalling pathway impact analysis; system biology; weighted gene co-expression network analysis.
Figures
References
-
- Pandžić JV, Grizelj D.. Under the surface of subcutaneous adipose tissue biology. Acta Dermatovenerol Croat. 2016. December;24(4):250–260. [Crossref], [PubMed], [Web of Science], [Google Scholar]. - PubMed
-
- Sniderman AD, Bhopal R, Prabhakaran D, et al. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007. January;36(1):220–225. [Crossref], [PubMed], [Web of Science], [Google Scholar]. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases