Analytical validation of CanAssist-Breast: an immunohistochemistry based prognostic test for hormone receptor positive breast cancer patients
- PMID: 30894144
- PMCID: PMC6425559
- DOI: 10.1186/s12885-019-5443-5
Analytical validation of CanAssist-Breast: an immunohistochemistry based prognostic test for hormone receptor positive breast cancer patients
Abstract
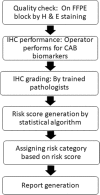
Background: CanAssist-Breast is an immunohistochemistry based test that predicts risk of distant recurrence in early-stage hormone receptor positive breast cancer patients within first five years of diagnosis. Immunohistochemistry gradings for 5 biomarkers (CD44, ABCC4, ABCC11, N-Cadherin and pan-Cadherins) and 3 clinical parameters (tumor size, tumor grade and node status) of 298 patient cohort were used to develop a machine learning based statistical algorithm. The algorithm generates a risk score based on which patients are stratified into two groups, low- or high-risk for recurrence. The aim of the current study is to demonstrate the analytical performance with respect to repeatability and reproducibility of CanAssist-Breast.
Methods: All potential sources of variation in CanAssist-Breast testing involving operator, run and observer that could affect the immunohistochemistry performance were tested using appropriate statistical analysis methods for each of the CanAssist-Breast biomarkers using a total 309 samples. The cumulative effect of these variations in the immunohistochemistry gradings on the generation of CanAssist-Breast risk score and risk category were also evaluated. Intra-class Correlation Coefficient, Bland Altman plots and pair-wise agreement were performed to establish concordance on IHC gradings, risk score and risk categorization respectively.
Results: CanAssist-Breast test exhibited high levels of concordance on immunohistochemistry gradings for all biomarkers with Intra-class Correlation Coefficient of ≥0.75 across all reproducibility and repeatability experiments. Bland-Altman plots demonstrated that agreement on risk scores between the comparators was within acceptable limits. We also observed > 90% agreement on risk categorization (low- or high-risk) across all variables tested.
Conclusions: The extensive analytical validation data for the CanAssist-Breast test, evaluating immunohistochemistry performance, risk score generation and risk categorization showed excellent agreement across variables, demonstrating that the test is robust.
Keywords: Analytical validation; CanAssist-breast; Immunohistochemistry; Repeatability; Reproducibility.
Conflict of interest statement
Ethics approval and consent to participate
Clearance for the samples used in this study has been obtained from the Bangalore ethical committee (ECR/87/Indt/KA/2013) and in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors are employees/consultants at OncoStem Diagnostics Private Limited which has developed the CanAssist-Breast test. MMB and CR are co-inventors on a patent application related to this article. All other authors have no other competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Fisher B, Jeong J-H, Bryant J, Anderson S, Dignam J. Fisher, et al. treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and bowel project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. - DOI - PubMed
-
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

