Targeting pericytes for neurovascular regeneration
- PMID: 30894190
- PMCID: PMC6425710
- DOI: 10.1186/s12964-019-0340-8
Targeting pericytes for neurovascular regeneration
Abstract
Pericytes, as a key cellular part of the blood-brain barrier, play an important role in the maintenance of brain neurovascular unit. These cells participate in brain homeostasis by regulating vascular development and integrity mainly through secreting various factors. Pericytes per se show different restorative properties after blood-brain barrier injury. Upon the occurrence of brain acute and chronic diseases, pericytes provoke immune cells to regulate neuro-inflammatory conditions. Loss of pericytes in distinct neurologic disorders intensifies blood-brain barrier permeability and leads to vascular dementia. The therapeutic potential of pericytes is originated from the unique morphological shape, location, and their ability in providing vast paracrine and juxtacrine interactions. A subset of pericytes possesses multipotentiality and exhibit trans-differentiation capacity in the context of damaged tissue. This review article aimed to highlight the critical role of pericytes in restoration of the blood-brain barrier after injury by focusing on the dynamics of pericytes and cross-talk with other cell types.
Keywords: Angiogenesis potential; Blood-brain barrier restoration; Pericytes.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
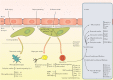
Similar articles
-
Stem Cells as a Promising Tool for the Restoration of Brain Neurovascular Unit and Angiogenic Orientation.Mol Neurobiol. 2017 Dec;54(10):7689-7705. doi: 10.1007/s12035-016-0286-4. Epub 2016 Nov 14. Mol Neurobiol. 2017. PMID: 27844280 Review.
-
Role of the CNS microvascular pericyte in the blood-brain barrier.J Neurosci Res. 1998 Sep 15;53(6):637-44. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. J Neurosci Res. 1998. PMID: 9753191 Review.
-
Brain microvascular pericytes in health and disease.Acta Neuropathol. 2011 Jul;122(1):1-9. doi: 10.1007/s00401-011-0847-6. Epub 2011 Jun 9. Acta Neuropathol. 2011. PMID: 21656168 Review.
-
Neurovascular unit: a focus on pericytes.Mol Neurobiol. 2012 Apr;45(2):327-47. doi: 10.1007/s12035-012-8244-2. Epub 2012 Feb 28. Mol Neurobiol. 2012. PMID: 22371274 Review.
-
Cell biology of the neurovascular unit: implications for drug delivery across the blood-brain barrier.Assay Drug Dev Technol. 2005 Feb;3(1):89-95. doi: 10.1089/adt.2005.3.89. Assay Drug Dev Technol. 2005. PMID: 15798399 Review.
Cited by
-
Pathological pericyte expansion and impaired endothelial cell-pericyte communication in endothelial Rbpj deficient brain arteriovenous malformation.Front Hum Neurosci. 2022 Sep 6;16:974033. doi: 10.3389/fnhum.2022.974033. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36147294 Free PMC article.
-
The role of mitochondrial transfer via tunneling nanotubes in the central nervous system: A review.Medicine (Baltimore). 2024 Mar 1;103(9):e37352. doi: 10.1097/MD.0000000000037352. Medicine (Baltimore). 2024. PMID: 38428884 Free PMC article. Review.
-
Vascular organs-on-chip made with patient-derived endothelial cells: technologies to transform drug discovery and disease modeling.Expert Opin Drug Discov. 2024 Mar;19(3):339-351. doi: 10.1080/17460441.2023.2294947. Epub 2023 Dec 20. Expert Opin Drug Discov. 2024. PMID: 38117223 Free PMC article. Review.
-
The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health.Cells. 2022 Oct 2;11(19):3108. doi: 10.3390/cells11193108. Cells. 2022. PMID: 36231071 Free PMC article. Review.
-
Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease.Ageing Res Rev. 2021 Jul;68:101335. doi: 10.1016/j.arr.2021.101335. Epub 2021 Mar 31. Ageing Res Rev. 2021. PMID: 33812051 Free PMC article. Review.
References
-
- Johnsen KB, Burkhart A, Melander F, Kempen PJ, Vejlebo JB, Siupka P, Nielsen MS, Andresen TL, Moos T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci Rep. 2017;7:10396. doi: 10.1038/s41598-017-11220-1. - DOI - PMC - PubMed
-
- Feng L, Sharma A, Niu F, Huang Y, Lafuente JV, Muresanu DF, Ozkizilcik A, Tian ZR, Sharma HS. TiO2-Nanowired delivery of DL-3-n-butylphthalide (DL-NBP) attenuates blood-brain barrier disruption, brain edema formation, and neuronal damages following concussive head injury. Mol Neurobiol. 2017. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

