The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients
- PMID: 30894219
- PMCID: PMC6425717
- DOI: 10.1186/s13075-019-1860-8
The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients
Abstract
Background: ABCG2 is a high-capacity urate transporter that plays a crucial role in renal urate overload and extra-renal urate underexcretion. Previous studies have suggested an association between hyperuricemia and gout susceptibility relative to dysfunctional ABCG2 variants, with rs2231142 (Q141K) being the most common. In this study, we analyzed the ABCG2 gene in a hyperuricemia and gout cohort focusing on patients with pediatric-onset, i.e., before 18 years of age.
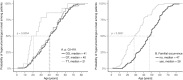
Method: The cohort was recruited from the Czech Republic (n = 234) and consisted of 58 primary hyperuricemia and 176 gout patients, with a focus on pediatric-onset patients (n = 31, 17 hyperuricemia/14 gouts); 115 normouricemic controls were used for comparison. We amplified, sequenced, and analyzed 15 ABCG2 exons. The chi-square goodness-of-fit test was used to compare minor allele frequencies (MAF), and the log-rank test was used to compare empirical distribution functions.
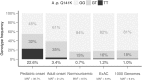
Results: In the pediatric-onset cohort, two common (p.V12M, p.Q141K) and three very rare (p.K360del, p.T421A, p.T434M) allelic ABCG2 variants were detected. The MAF of p.Q141K was 38.7% compared to adult-onset MAF 21.2% (OR = 2.4, P = 0.005), to the normouricemic controls cohort MAF 8.5% (OR = 6.8, P < 0.0001), and to the European population MAF 9.4% (OR = 5.7, P < 0.0001). The MAF was greatly elevated not only among pediatric-onset gout patients (42.9%) but also among patients with hyperuricemia (35.3%). Most (74%) of the pediatric-onset patients had affected family members (61% were first-degree relatives).
Conclusion: Our results show that genetic factors affecting ABCG2 function should be routinely considered in a hyperuricemia/gout diagnosis, especially in pediatric-onset patients. Genotyping of ABCG2 is essential for risk estimation of gout/hyperuricemia in patients with very early-onset and/or a family history.
Keywords: ABCG2; Gout; Hyperuricemia; Urate transport.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Institute of Rheumatology (reference number 6181/2015). All patients and healthy controls were fully informed of the aim of the study, and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Identification of Two Dysfunctional Variants in the ABCG2 Urate Transporter Associated with Pediatric-Onset of Familial Hyperuricemia and Early-Onset Gout.Int J Mol Sci. 2021 Feb 16;22(4):1935. doi: 10.3390/ijms22041935. Int J Mol Sci. 2021. PMID: 33669292 Free PMC article.
-
Functional non-synonymous variants of ABCG2 and gout risk.Rheumatology (Oxford). 2017 Nov 1;56(11):1982-1992. doi: 10.1093/rheumatology/kex295. Rheumatology (Oxford). 2017. PMID: 28968913
-
Functional Characterization of Clinically-Relevant Rare Variants in ABCG2 Identified in a Gout and Hyperuricemia Cohort.Cells. 2019 Apr 18;8(4):363. doi: 10.3390/cells8040363. Cells. 2019. PMID: 31003562 Free PMC article.
-
ABCG2 as a therapeutic target candidate for gout.Expert Opin Ther Targets. 2018 Feb;22(2):123-129. doi: 10.1080/14728222.2018.1420167. Epub 2017 Dec 28. Expert Opin Ther Targets. 2018. PMID: 29264928 Review.
-
The association between genetic polymorphisms in ABCG2 and SLC2A9 and urate: an updated systematic review and meta-analysis.BMC Med Genet. 2020 Oct 21;21(1):210. doi: 10.1186/s12881-020-01147-2. BMC Med Genet. 2020. PMID: 33087043 Free PMC article.
Cited by
-
Genetic polymorphisms and decreased protein expression of ABCG2 urate transporters are associated with susceptibility to gout, disease severity and renal-overload hyperuricemia.Clin Exp Med. 2023 Aug;23(4):1277-1284. doi: 10.1007/s10238-022-00848-7. Epub 2022 Aug 8. Clin Exp Med. 2023. PMID: 35939175 Free PMC article.
-
High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout.Int J Mol Sci. 2020 Mar 20;21(6):2147. doi: 10.3390/ijms21062147. Int J Mol Sci. 2020. PMID: 32245084 Free PMC article.
-
Expression, Function and Trafficking of the Human ABCG2 Multidrug Transporter Containing Mutations in an Unstructured Cytoplasmic Loop.Membranes (Basel). 2023 Oct 4;13(10):822. doi: 10.3390/membranes13100822. Membranes (Basel). 2023. PMID: 37887994 Free PMC article.
-
3D structure of the transporter ABCG2-What's new?Br J Pharmacol. 2020 Apr;177(7):1485-1496. doi: 10.1111/bph.14991. Epub 2020 Feb 11. Br J Pharmacol. 2020. PMID: 31985041 Free PMC article. Review.
-
The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout-An Update.Int J Mol Sci. 2021 Jun 22;22(13):6678. doi: 10.3390/ijms22136678. Int J Mol Sci. 2021. PMID: 34206432 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

