Tks5 and Dynamin-2 enhance actin bundle rigidity in invadosomes to promote myoblast fusion
- PMID: 30894403
- PMCID: PMC6504888
- DOI: 10.1083/jcb.201809161
Tks5 and Dynamin-2 enhance actin bundle rigidity in invadosomes to promote myoblast fusion
Abstract
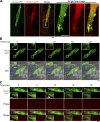
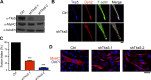
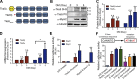
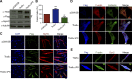
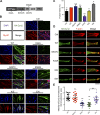
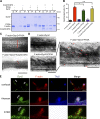
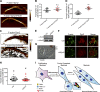
Skeletal muscle development requires the cell-cell fusion of differentiated myoblasts to form muscle fibers. The actin cytoskeleton is known to be the main driving force for myoblast fusion; however, how actin is organized to direct intercellular fusion remains unclear. Here we show that an actin- and dynamin-2-enriched protrusive structure, the invadosome, is required for the fusion process of myogenesis. Upon differentiation, myoblasts acquire the ability to form invadosomes through isoform switching of a critical invadosome scaffold protein, Tks5. Tks5 directly interacts with and recruits dynamin-2 to the invadosome and regulates its assembly around actin filaments to strengthen the stiffness of dynamin-actin bundles and invadosomes. These findings provide a mechanistic framework for the acquisition of myogenic fusion machinery during myogenesis and reveal a novel structural function for Tks5 and dynamin-2 in organizing actin filaments in the invadosome to drive membrane fusion.
© 2019 Chuang et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

